Drug Catalog - Product Detail
FLUOCINONIDE - E CRM 0.05% 30G
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1706-45 | AMNEAL PHARMACEUTICALS | 30 | 0.05% | CREAM |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
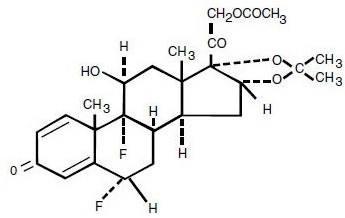
DESCRIPTION Fluocinonide Cream, USP 0.05% (Emulsified Base) is intended for topical administration. The active component is the corticosteroid fluocinonide, which is the 21-acetate ester of fluocinolone acetonide and has the chemical name pregna-1,4-diene-3,20-dione,21-(acetyloxy)-6,9-difluoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (6α ,11β,16α )-. It has the following chemical structure: Mol. Formula: C 26 H 32 F 2 O 7 Mol. Wt: 494.53 Fluocinonide Cream, USP 0.05% (Emulsified Base) contains fluocinonide 0.5 mg/g in a water-washable aqueous emollient base of cetyl alcohol, citric acid (anhydrous), mineral oil, polysorbate 60, propylene glycol, purified water, sorbitan monostearate, stearyl alcohol.
How Supplied
HOW SUPPLIED Fluocinonide Cream, USP 0.05% (Emulsified Base) is supplied in 15 g (NDC 0115-1705-52), 30 g (NDC 0115-1706-45) and 60 g (NDC 0115-1707-58) Tubes. Store at controlled room temperature 15°-30°C (59°-86°F). Do not refrigerate. Manufactured by: G&W Laboratories, Inc. 111 Coolidge Street South Plainfield, NJ 07080 Distributed by: Impax Generics Hayward, CA 94544 1933-01 GW 7140 Issued 08/2016 8-0664IMLNC1
Indications & Usage
INDICATIONS AND USAGE Fluocinonide Cream USP, 0.05% (Emulsified Base) is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
Dosage and Administration
DOSAGE AND ADMINISTRATION Fluocinonide Cream, USP 0.05% (Emulsified Base) is generally applied to the affected area as a thin film from two to four times daily, as needed. Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions. If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
