Drug Catalog - Product Detail
FLUOCINOLONE ACETONIDE TOPICAL OIL (SCALP OIL) OIL 0.0001 118ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0703-86 | AMNEAL PHARMACEUTICALS | 118 | 0.01% | OIL |
PACKAGE FILES

Generic Name
FLUOCINOLONE ACETONIDE
Substance Name
FLUOCINOLONE ACETONIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA201759
Description
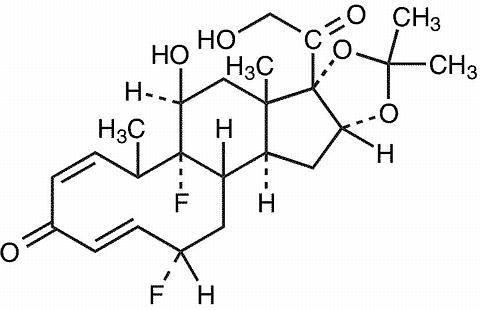
DESCRIPTION Fluocinolone Acetonide 0.01 % Topical Oil contains fluocinolone acetonide {(6α, 11β, 16α)-6,9-difluoro-11,21-dihydroxy-16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic 16,17 acetal with acetone}, a synthetic corticosteroid for topical use dermatologic use. This formulation is also marketed as Fluocinolone Acetonide 0.01% Topical Oil for use as body oil for atopic dermatitis in adults and for moderate to severe atopic dermatitis in pediatric patients 2 years and older, and as fluocinolone acetonide oil, 0.01% for chronic eczematous external otitis. Chemically, fluocinolone acetonide is C 24 H 30 F 2 O 6 . It has the following structural formula: Fluocinolone acetonide in Fluocinolone Acetonide 0.01% Topical Oil has a molecular weight of 452.50. It is a white crystalline powder that is odorless, stable in light, and melts at 270ºC with decomposition; soluble in alcohol, acetone and methanol; slightly soluble in chloroform; insoluble in water. Each gram of Fluocinolone Acetonide 0.01% Topical Oil contains approximately 0.11 mg of fluocinolone acetonide in a blend of oils, which contains isopropyl alcohol, isopropyl myristate, light mineral oil, oleth-2, and refined peanut oil NF. Each packaged product contains 2 shower caps. The shower cap is made of low density polyethylene material with rubber elastic. 32207a82-figure-01
How Supplied
HOW SUPPLIED Fluocinolone Acetonide 0.01% Topical Oil is supplied in a 4 fluid ounce bottle with a net content of 118.28 mL. It is labeled as Scalp Oil (NDC 65162-703-86). Scalp Oil is supplied with 2 shower caps. Keep tightly closed. Store upright at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or www.amneal.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. CAUTION: Rx only Distributed by: Amneal Pharmaceuticals Bridgewater, NJ 08807 Rev. 08-2015-00
Indications & Usage
INDICATIONS AND USAGE Fluocinolone Acetonide 0.01% Topical Oil is a low to medium potency corticosteroid indicated: In adult patients for the treatment of psoriasis of the scalp (Scalp Oil).
Dosage and Administration
DOSAGE AND ADMINISTRATION Fluocinolone Acetonide 0.01% Topical Oil for scalp psoriasis in adults (Scalp Oil): For the treatment of scalp psoriasis, wet or dampen hair and scalp thoroughly. Apply a thin film of Fluocinolone Acetonide 0.01% Topical Oil on the scalp, massage well and cover scalp with the supplied shower cap. Leave on overnight, or for a minimum of 4 hours before washing off. Wash hair with regular shampoo and rinse thoroughly.
