Drug Catalog - Product Detail
FLUDROCORTISONE ACETATE TB 0.1MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-7033-01 | AMNEAL PHARMACEUTICALS | 100 | 0.1MG | TABLET |
PACKAGE FILES


Generic Name
FLUDROCORTISONE ACETATE
Substance Name
FLUDROCORTISONE ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040431
Description
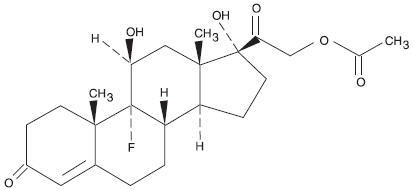
DESCRIPTION Fludrocortisone acetate tablets USP, 0.1 mg contain fludrocortisone acetate, USP a synthetic adrenocortical steroid possessing very potent mineralocorticoid properties and high glucocorticoid activity; it is used only for its mineralocorticoid effects. The chemical name for fludrocortisone acetate is 9-fluoro-11β, 17, 21-trihydroxypregn-4-ene-3, 20-dione 21-acetate; its structural formula is: C 23 H 31 FO 6 MW 422.49 Fludrocortisone acetate tablets USP, 0.1 mg are available for oral administration as scored tablets providing 0.1 mg fludrocortisone acetate, USP per tablet. Inactive ingredients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, and microcrystalline cellulose NF. Chemical Structure
How Supplied
HOW SUPPLIED Fludrocortisone Acetate Tablets USP, 0.1 mg — Each white to off-white, round, convex tablet debossed with a “7033” on one side and with a bisect on the other side. They are available as follows: Bottles of 100: NDC 0115-7033-01 Bottles of 500: NDC 0115-7033-02 Bottles of 1000: NDC 0115-7033-03 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Avoid excessive heat. Dispense in a tightly-closed, light-resistant container (USP). Manufactured by: Amneal Pharmaceuticals Pvt. Ltd. Ahmedabad 382220, INDIA Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807 Rev. 08-2024-03
Indications & Usage
INDICATIONS AND USAGE Fludrocortisone acetate tablets, 0.1 mg are indicated as partial replacement therapy for primary and secondary adrenocortical insufficiency in Addison’s disease and for the treatment of salt-losing adrenogenital syndrome.
Dosage and Administration
DOSAGE AND ADMINISTRATION Dosage depends on the severity of the disease and the response of the patient. Patients should be continually monitored for signs that indicate dosage adjustment is necessary, such as remission or exacerbations of the disease and stress (surgery, infection, trauma) (see WARNINGS and PRECAUTIONS, General ). Addison's Disease In Addison’s disease, the combination of fludrocortisone acetate tablets with a glucocorticoid such as hydrocortisone or cortisone provides substitution therapy approximating normal adrenal activity with minimal risks of unwanted effects. The usual dose is 0.1 mg of fludrocortisone acetate tablets daily, although dosage ranging from 0.1 mg three times a week to 0.2 mg daily has been employed. In the event transient hypertension develops as a consequence of therapy, the dose should be reduced to 0.05 mg daily. Fludrocortisone acetate tablets are preferably administered in conjunction with cortisone (10 mg to 37.5 mg daily in divided doses) or hydrocortisone (10 mg to 30 mg daily in divided doses). Salt-Losing Adrenogenital Syndrome The recommended dosage for treating the salt-losing adrenogenital syndrome is 0.1 mg to 0.2 mg of fludrocortisone acetate tablets daily.
