Drug Catalog - Product Detail
Finasteride Tab 1 MG 90 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 67877-0455-90 | ASCEND LABORATORIES | 90 | 1MG | TABLET |
PACKAGE FILES


Generic Name
FINASTERIDE
Substance Name
FINASTERIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207750
Description
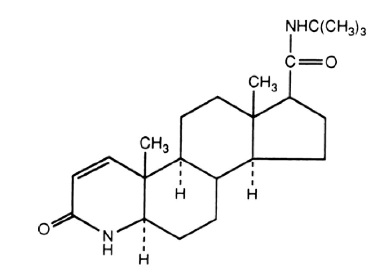
11 DESCRIPTION Finasteride tablets, USP contain finasteride as the active ingredient. Finasteride, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT). The chemical name of finasteride is N-tert -Butyl-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide. The empirical formula of finasteride is C 23 H 36 N 2 O 2 and its molecular weight is 372.55. Its structural formula is: Finasteride is a white crystalline powder with a melting point near 250°C. It is freely soluble in chloroform and in lower alcohol solvents but is practically insoluble in water. Finasteride Tablets, USP are film-coated tablets for oral administration. Each tablet contains 1 mg of finasteride and the following inactive ingredients: hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, lauroylmacrogol 32 glycerides, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch, sodium starch glycolate, and titanium dioxide. finasteride-structure.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Finasteride tablets, USP 1 mg are available as reddish brown colored, 7 mm round, biconvex, film coated tablets, marked “F1” on one side and plain on other side. They are supplied as follows: 30’s HDPE Container pack: NDC 67877-455-30 90’s HDPE Container pack: NDC 67877-455-90 500’s HDPE Container pack: NDC 67877-455-05 Carton of 30 (3 x 10 unit-dose) Tablets: NDC 67877-455-94 Storage and Handling Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Preserve in a tight, light-resistant container. Women should not handle crushed or broken finasteride tablets when they are pregnant or may potentially be pregnant because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus. Finasteride tablets, USP 1 mg are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets are not broken or crushed [ see Warnings and Precautions (5.1) , Use in Specific Populations (8.1) and Patient Counseling Information (17)] .
Indications & Usage
1 INDICATIONS AND USAGE Finasteride tablets is indicated for the treatment of male pattern hair loss (androgenetic alopecia) in MEN ONLY . Efficacy in bitemporal recession has not been established. Finasteride tablets is not indicated for use in women. Finasteride tablets is a 5α-reductase inhibitor indicated for the treatment of male pattern hair loss (androgenetic alopecia) in MEN ONLY ( 1 ). Finasteride tablets is not indicated for use in women ( 1 , 4 , 5.1 ).
Dosage and Administration
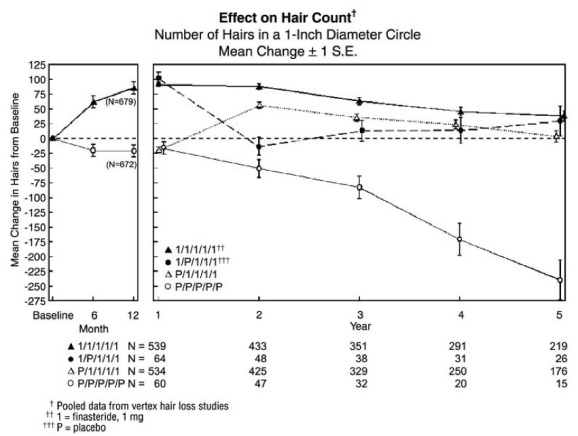
2 DOSAGE AND ADMINISTRATION Finasteride tablets may be administered with or without meals. The recommended dose of finasteride tablets, USP 1 mg is one tablet (1 mg) taken once daily. In general, daily use for three months or more is necessary before benefit is observed. Continued use is recommended to sustain benefit, which should be re-evaluated periodically. Withdrawal of treatment leads to reversal of effect within 12 months. Finasteride tablets may be administered with or without meals (2). One tablet ( 1 mg ) taken once daily ( 2 ). In general, daily use for three months or more is necessary before benefit is observed ( 2 ).
