Drug Catalog - Product Detail
FENOFIBRATE MICRONIZED CAP 130 MG 30 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 63304-0444-30 | SUN PHARMACEUTICALS | 30 | 130MG | CAPSULE |
PACKAGE FILES
Generic Name
FENOFIBRATE
Substance Name
FENOFIBRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA201748
Description
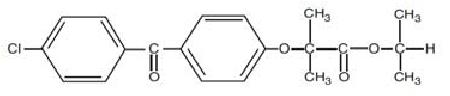
11 DESCRIPTION Fenofibrate capsules, USP, is a lipid regulating agent available as capsules for oral administration. Each capsule contains 43 mg or 130 mg of fenofibrate (micronized), USP. The chemical name for fenofibrate is 2-[4-(4-chlorobenzoyl) phenoxy]-2-methyl-propanoic acid, l-methylethyl ester with the following structural formula: The molecular formula is C 20 H 21 O 4 Cl and the molecular weight is 360.83; fenofibrate (micronized), USP is practically insoluble in water, very soluble in methylene chloride, slightly soluble in alcohol. The melting point is 79° to 82° C. Fenofibrate (micronized), USP is a white or almost white crystalline powder. Inactive Ingredients: Each gelatin capsule contains hypromellose, pregelatinized starch (maize), simethicone emulsion, sodium lauryl sulfate, sodium stearyl fumarate and talc. The gelatin capsules also contain gelatin and titanium dioxide. The imprinting ink contains ferric oxide black, potassium hydroxide, propylene glycol, and shellac. Meets USP Dissolution Test 2. Structure
How Supplied
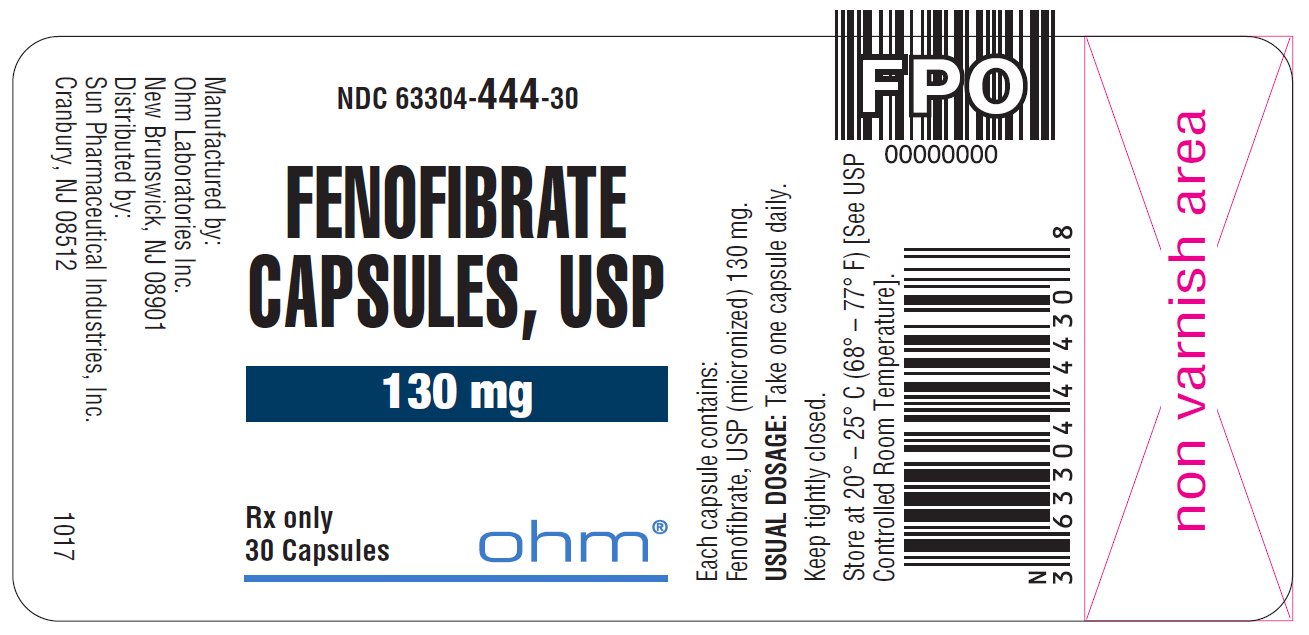
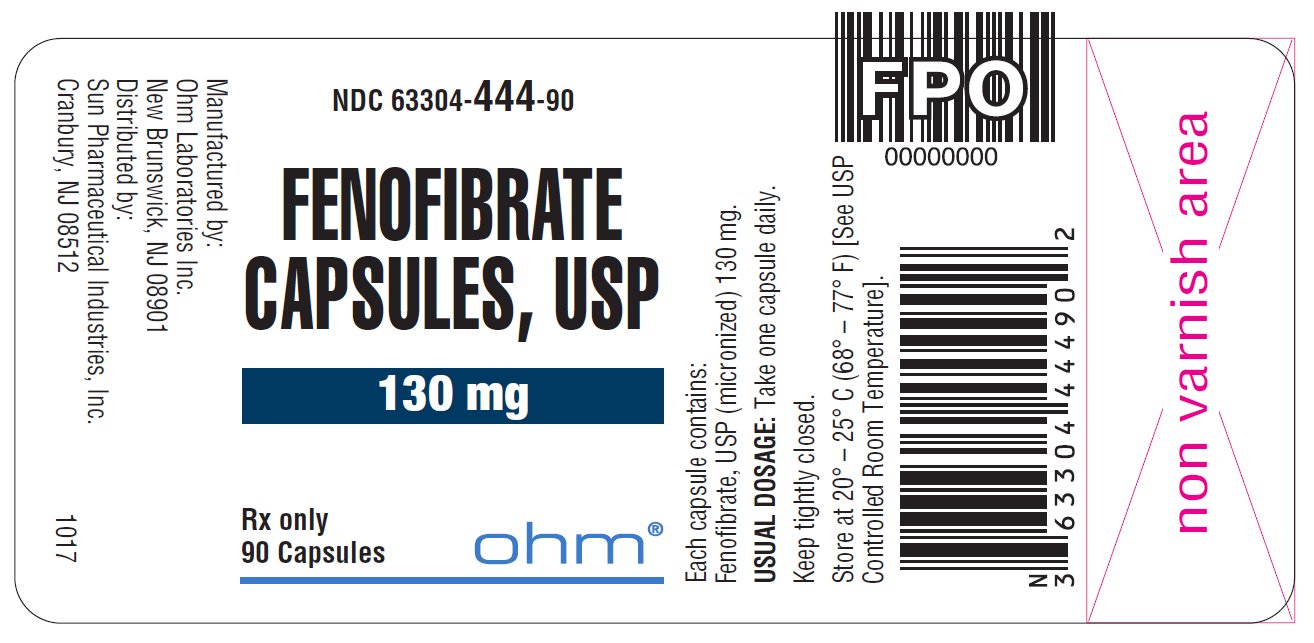
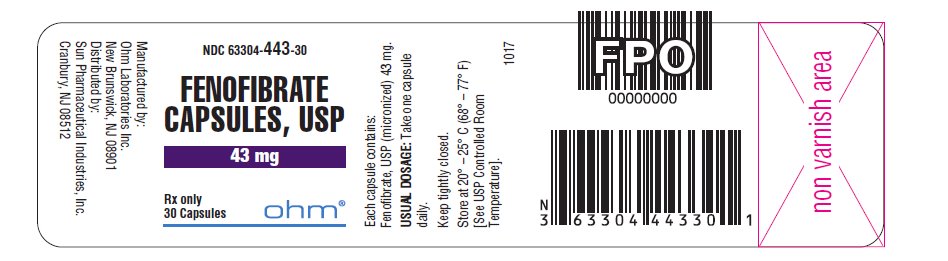
16 HOW SUPPLIED/STORAGE AND HANDLING Fenofibrate capsules, USP, are available in two strengths: • 43 mg capsules, white to off-white granular powder filled in size ‘4’ white opaque cap and white opaque body hard gelatin capsule imprinted with ‘ RG78 ’ on cap and body in black ink. NDC 63304-443-30 Bottles of 30 NDC 63304-443-05 Bottles of 500 • 130 mg capsules, white to off-white granular powder filled in size ‘0’ white opaque cap and white opaque body hard gelatin capsule imprinted with ‘ RG79 ’ on cap and body in black ink. NDC 63304-444-30 Bottles of 30 NDC 63304-444-90 Bottles of 90 NDC 63304-444-05 Bottles of 500 Storage: Store at 20º - 25º C (68º - 77º F) [See USP Controlled Room Temperature] in a tightly closed container.
Indications & Usage
1 INDICATIONS AND USAGE Fenofibrate capsules, USP are peroxisome proliferator receptor alpha (PPARα) activator indicated as an adjunct to diet: • to reduce elevated LDL-C, Total-C, triglycerides, and Apo B, and to increase HDL-C in adult patients with primary hypercholesterolemia or mixed dyslipidemia (1.1). • to reduce triglyceride (TG) levels in adult patients with severe hypertriglyceridemia (1.2). Important Limitations of Use: Fenofibrate was not shown to reduce coronary heart disease morbidity and mortality in patients with type 2 diabetes mellitus (5.1). 1.1 Primary Hypercholesterolemia and Mixed Dyslipidemia Fenofibrate capsules, USP are indicated as adjunctive therapy to diet to reduce elevated low-density lipoprotein cholesterol (LDL-C), total cholesterol (Total-C), triglycerides (TG), and apolipoprotein B (Apo B), and to increase high-density lipoprotein cholesterol (HDL-C) in adult patients with primary hypercholesterolemia or mixed dyslipidemia. 1.2 Severe Hypertriglyceridemia Fenofibrate capsules, USP are also indicated as adjunctive therapy to diet for treatment of adult patients with severe hypertriglyceridemia. Improving glycemic control in diabetic patients showing fasting chylomicronemia will usually reduce fasting triglycerides and eliminate chylomicronemia thereby obviating the need for pharmacologic intervention. Markedly elevated levels of serum triglycerides (e.g > 2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of fenofibrate therapy on reducing this risk has not been adequately studied. 1.3 Important Limitations of Use Fenofibrate was not shown to reduce coronary heart disease morbidity and mortality in patients with type 2 diabetes mellitus [see Warnings and Precautions (5.1)] .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Fenofibrate capsules can be taken without regard to meals (2.1). • Primary hypercholesterolemia and mixed dyslipidemia: 130 mg per day (2.2). • Severe Hypertriglyceridemia: 43 to 130 mg per day; the dose should be adjusted according to patient response (2.3). • Renally impaired patients: Initial dose of 43 mg per day (2.4). • Geriatric patients: Select the dose on the basis of renal function (2.5). 2.1 General Considerations Patients should be placed on an appropriate lipid-lowering diet before receiving fenofibrate capsules, and should continue this diet during treatment with fenofibrate capsules. Fenofibrate capsules can be given without regard to meals. Patients should be advised to swallow fenofibrate capsules whole. Do not open, crush, dissolve or chew capsules. The initial treatment for dyslipidemia is dietary therapy specific for the type of lipoprotein abnormality. Excess body weight and excess alcoholic intake may be important factors in hypertriglyceridemia and should be addressed prior to any drug therapy. Physical exercise can be an important ancillary measure. Diseases contributory to hyperlipidemia, such as hypothyroidism or diabetes mellitus should be looked for and adequately treated. Estrogen therapy, thiazide diuretics and beta-blockers are sometimes associated with massive rises in plasma triglycerides, especially in subjects with familial hypertriglyceridemia. In such cases, discontinuation of the specific etiologic agent may obviate the need for specific drug therapy of hypertriglyceridemia . Lipid levels should be monitored periodically and consideration should be given to reducing the dosage of fenofibrate capsules if lipid levels fall significantly below the targeted range. Therapy should be withdrawn in patients who do not have an adequate response after two months of treatment with the maximum recommended dose of 130 mg once daily. 2.2 Primary Hypercholesterolemia and Mixed Dyslipidemia The initial dose of fenofibrate capsules is 130 mg per day. 2.3 Severe Hypertriglyceridemia The initial dose is 43 to 130 mg per day. Dosage should be individualized according to patient response, and should be adjusted if necessary following repeat lipid determinations at 4 to 8 week intervals. The maximum dose is 130 mg per day. 2.4 Impaired Renal Function Treatment with fenofibrate capsules should be initiated at a dose of 43 mg per day in patients having mild to moderately impaired renal function, and increased only after evaluation of the effects on renal function and lipid levels at this dose. The use of fenofibrate capsules should be avoided in patients with severe renal impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] . 2.5 Geriatric Patients Dose selection for the elderly should be made on the basis of renal function [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] .
