Drug Catalog - Product Detail
FAMOTIDINE TB 20MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
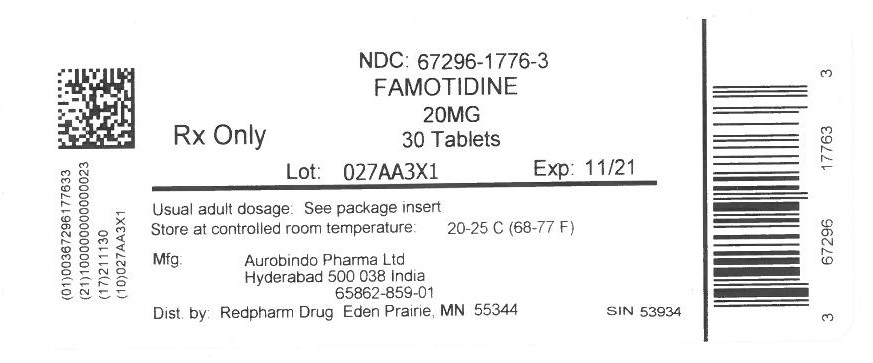
| 65862-0859-01 | AUROBINDO PHARMA | 100 | 20MG | TABLET |
PACKAGE FILES

Generic Name
FAMOTIDINE
Substance Name
FAMOTIDINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA206530
Description
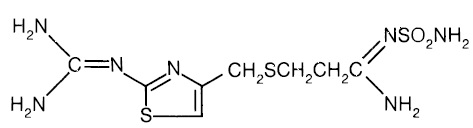
11 DESCRIPTION The active ingredient in famotidine tablets USP is a histamine-2 (H 2 ) receptor antagonist. Famotidine is N ′(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio] propanimidamide. The molecular formula of famotidine is C 8 H 15 N 7 O 2 S 3 and its molecular weight is 337.43. Its structural formula is: Each famotidine tablet for oral administration contains either 20 mg or 40 mg of famotidine USP and the following inactive ingredients: carnauba wax, corn starch, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, talc, and titanium dioxide. In addition the 20 mg tablets contain red iron oxide, and yellow iron oxide. Famotidine USP is a white to pale yellowish white crystalline powder that is freely soluble in glacial acetic acid, slightly soluble in methanol, very slightly soluble in water, and practically insoluble in ethanol. str1
How Supplied
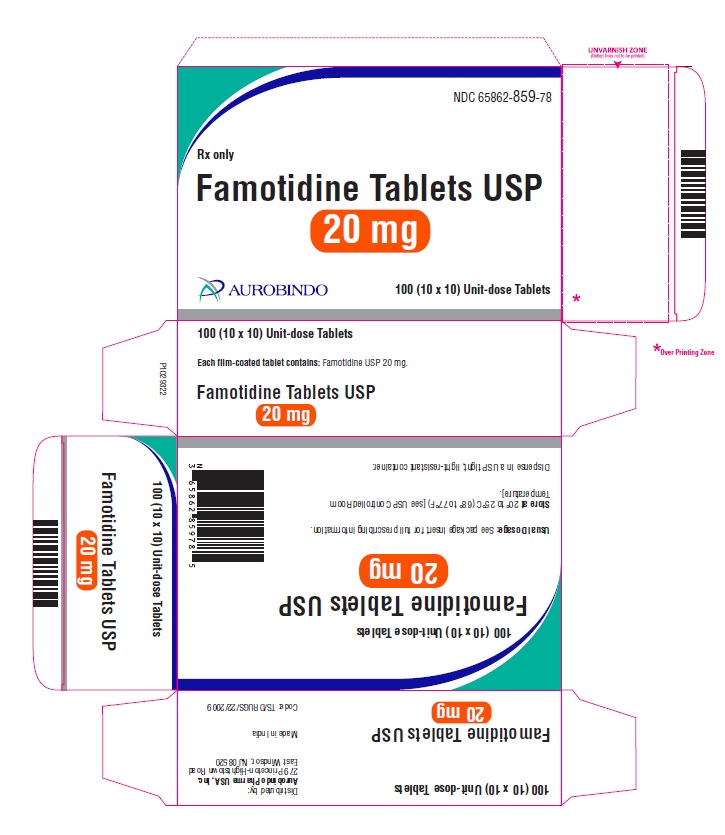
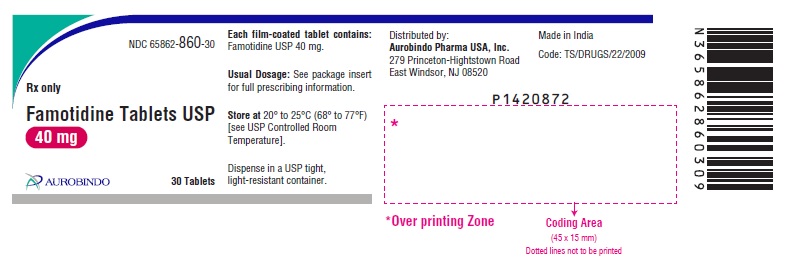
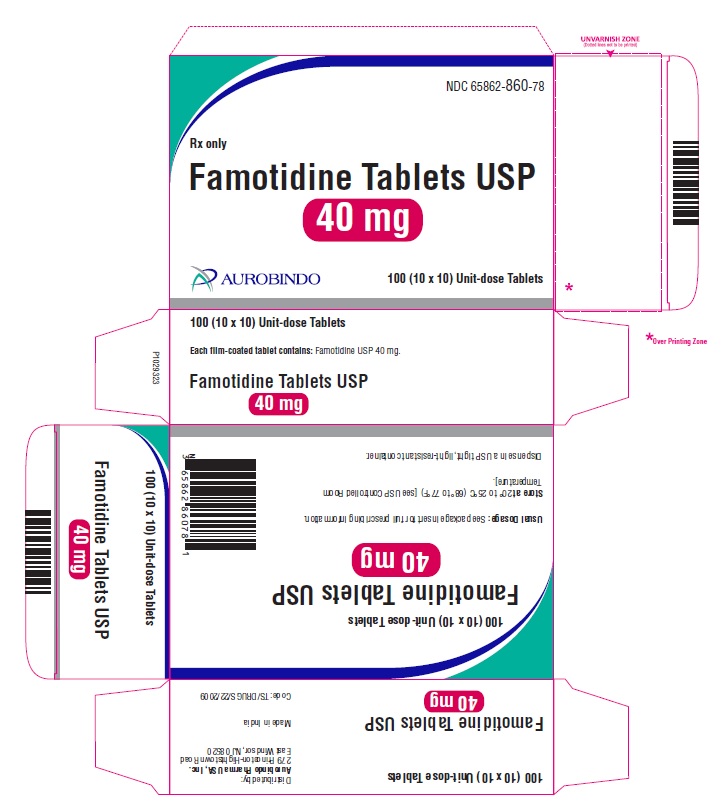
16 HOW SUPPLIED/STORAGE AND HANDLING Famotidine Tablets USP, 20 mg are yellow, rounded square shaped, biconvex, film-coated tablets debossed with ‘CC’ on one side and ‘60’ on the other side. Bottles of 30 NDC 65862-859-30 Bottles of 100 NDC 65862-859-01 Bottles of 500 NDC 65862-859-05 Bottles of 1,000 NDC 65862-859-99 10 x 10 Unit-dose Tablets NDC 65862-859-78 Famotidine Tablets USP, 40 mg are white, rounded square shaped, biconvex, film-coated tablets debossed with ‘CC’ on one side and ‘61’ on the other side. Bottles of 30 NDC 65862-860-30 Bottles of 100 NDC 65862-860-01 Bottles of 500 NDC 65862-860-05 Bottles of 1,000 NDC 65862-860-99 10 x 10 Unit-dose Tablets NDC 65862-860-78 Storage Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a USP tight, light-resistant container.
Indications & Usage
1 INDICATIONS AND USAGE Famotidine tablets are indicated in adult and pediatric patients 40 kg and greater for the treatment of: active duodenal ulcer (DU). active gastric ulcer (GU). symptomatic nonerosive gastroesophageal reflux disease (GERD). erosive esophagitis due to GERD, diagnosed by biopsy. Famotidine tablets are indicated in adults for the: treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome, multiple endocrine neoplasias). reduction of the risk of duodenal ulcer recurrence.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage Table 1 shows the recommended dosage of famotidine 20 mg and 40 mg tablets in adult and pediatric patients weighing 40 kg and greater with normal renal function. The use of famotidine 20 mg and 40 mg tablets is not recommended in pediatric patients weighing less than 40 kg because the lowest available strength (20 mg) exceeds the recommended dose for these patients . Use another famotidine formulation for pediatric patients weighing less than 40 kg. Table 1: Recommended Dosage and Duration of Famotidine Tablets in Adult and Pediatric Patients 40 kg and Greater with Normal Renal Function Indication Recommended Dosage Recommended Duration Active duodenal ulcer (DU) 40 mg once daily; or 20 mg twice daily a Up to 8 weeks b,c Active gastric ulcer 40 mg once daily Up to 8 weeks c Symptomatic nonerosive GERD 20 mg twice daily Up to 6 weeks c Erosive esophagitis diagnosed by endoscopy 20 mg twice daily; or 40 mg twice daily a Up to 12 weeks Pathological hypersecretory conditions d Starting dosage: 20 mg every 6 hours; adjust dosage to individual patient needs Maximum dosage 160 mg every 6 hours As clinically indicated Reduction of the risk of DU recurrence d 20 mg once daily 1 year c or as clinically indicated a Both dosages demonstrated effectiveness in clinical trials [see Clinical Studies (14) ] . b In clinical trials, the majority of patients healed within 4 weeks. For patients who do not heal after 4 weeks, consider an additional 2 to 4 weeks of treatment [see Clinical Studies (14.1) ]. c Longer treatment durations have not been studied in clinical trials [see Clinical Studies (14.1 , 14.2 , 14.3) ] . d In pediatric patients, the safety and effectiveness of famotidine tablets have not been established for the reduction of the risk of duodenal ulcer recurrence or for treatment of pathological hypersecretory conditions [see Use in Specific Populations (8.4) ] . 2.2 Dosage in Renal Impairment Dosage adjustments of famotidine tablets are recommended for patients with moderate to severe renal impairment (creatinine clearance less than 60 mL/min) [see Use in Specific Populations (8.6) ] . Table 2 shows the recommended maximum dosage of famotidine 20 mg or 40 mg tablets for patients with renal impairment, by indication. Use the lowest effective dose. Some dosage adjustments may require switching to other formulations of famotidine (e.g., oral suspension, lower dose tablet). Table 2: Recommended Maximum Dosage of Famotidine Tablets in Adult and Pediatric Patients 40 kg and Greater with Moderate and Severe Renal Impairment a An alternate dosage regimen is 10 mg once daily. Since 20 mg or 40 mg tablet strength cannot be used for this dosage regimen, use an alternate famotidine formulation. b Dosage adjustments for renal impairment are provided for both dosing regimens (20 mg twice daily and 40 mg twice daily) which showed effectiveness for the treatment of erosive esophagitis in clinical trials [see Clinical Studies (14.4) ] . c In pediatric patients, the safety and effectiveness of famotidine tablets have not been established for the reduction of the risk of duodenal ulcer recurrence or for treatment of pathological hypersecretory conditions [see Use in Specific Populations (8.4) ] . d Doses required to treat pathological hypersecretory conditions may exceed the maximum doses evaluated in patients with impaired renal function. The risk for increased adverse reactions in renally impaired patients treated with famotidine tablets for pathological hypersecretory conditions is unknown. e Recommended dosage regimen is 10 mg every other day. Since 20 mg or 40 mg tablet strength cannot be used for this dosage regimen, use an alternate famotidine formulation. Indication Recommended Maximum Dosages Creatinine clearance 30 to 60 mL/minute Creatinine clearance less than 30 mL/minute Active duodenal ulcer (DU) 20 mg once daily; or 40 mg every other day 20 mg every other day a Active gastric ulcer 20 mg once daily; or 40 mg every other day 20 mg every other day a Symptomatic nonerosive GERD 20 mg once daily 20 mg every other day a Erosive esophagitis diagnosed by endoscopy b 20 mg once daily; or 40 mg every other day b 20 mg every other day a,b 40 mg once daily b 20 mg once daily b Pathological hypersecretory conditions c Avoid use d Reduction of the risk of DU recurrence c 20 mg every other day a (see footnote) e 2.3 Administration Instructions Take famotidine tablets once daily before bedtime or twice daily in the morning and before bedtime, as recommended. Famotidine tablets may be taken with or without food [see Clinical Pharmacology (12.3) ] . Famotidine tablets may be given with antacids.
