Drug Catalog - Product Detail
ETODOLAC TAB ER 24HR 500 MG 60 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68382-0272-14 | ZYDUS PHARMACEUTICALS (USA) | 60 | 500MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
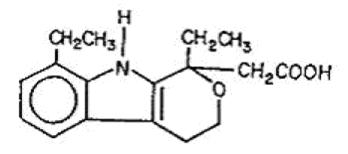
Description Etodolac extended-release tablets, USP contain etodolac, which is a member of the pyranocarboxylic acid group of non-steroidal anti-inflammatory drugs (NSAIDs). Each tablet contains etodolac for oral administration. Etodolac is a racemic mixture of [+]S and [-]R-enantiomers. Etodolac, USP is a white to off-white crystalline powder, insoluble in water but soluble in alcohols, chloroform, dimethyl sulfoxide, and aqueous polyethylene glycol. The chemical name is (±) 1,8-diethyl-1,3,4,9-tetrahydropyrano-[3,4-b]indole-1-acetic acid. It has the following structural formula: C17H21NO3 M.W. 287.36 Each etodolac extended-release tablet, USP intended for oral administration contains 400 mg or 500 mg or 600 mg of etodolac. In addition, each tablet contains the following inactive ingredients: disodium hydrogen phosphate, ethylcellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, talc, titanium dioxide and triacetin. Additionally each 400 mg tablet contains: D&C yellow # 10 aluminum lake, FD&C Red # 40 aluminum lake and FD&C yellow # 6 aluminum lake and each 500 mg tablet contains: FD&C Blue # 2 aluminum lake, iron oxide black and iron oxide yellow and each 600 mg tablet contains: FD&C blue # 2 aluminum lake, iron oxide red and iron oxide yellow. The USP Drug Release test complies with USP Dissolution Test 1. Chemical Structure
How Supplied
How Supplied Etodolac Extended-release Tablets USP, 400 mg are orange-colored, oval-shaped, beveled edged, film-coated tablets, debossed with "271" on one side and plain on other side and are supplied as follows: NDC 68382-271-14 in bottles of 60 tablets NDC 68382-271-01 in bottles of 100 tablets NDC 68382-271-05 in bottles of 500 tablets NDC 68382-271-10 in bottles of 1000 tablets NDC 68382-271-77 in unit-dose blister cartons of 100 (10 x 10) unit dose tablets Etodolac Extended-release Tablets USP, 500 mg are grey-colored, oval-shaped, beveled edged, film-coated tablets, debossed with "272" on one side and plain on other side and are supplied as follows: NDC 68382-272-14 in bottles of 60 tablets NDC 68382-272-01 in bottles of 100 tablets NDC 68382-272-05 in bottles of 500 tablets NDC 68382-272-10 in bottles of 1000 tablets NDC 68382-272-77 in unit-dose blister cartons of 100 (10 x 10) unit dose tablets Etodolac Extended-release Tablets USP, 600 mg are blue-colored, oval-shaped, beveled edged, film-coated tablets debossed with "273" on one side and plain on other side and are supplied as follows: NDC 68382-273-14 in bottles of 60 tablets NDC 68382-273-01 in bottles of 100 tablets NDC 68382-273-05 in bottles of 500 tablets NDC 68382-273-10 in bottles of 1000 tablets NDC 68382-273-77 in unit-dose blister cartons of 100 (10 x 10) unit dose tablets Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from excessive heat and humidity. Dispense in a tight, light-resistant container with a child-resistant closure.
Indications & Usage
Indications & Usage Carefully consider the potential benefits and risks of etodolac extended-release tablets, USP and other treatment options before deciding to use etodolac extended-release tablets, USP. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS). Etodolac extended-release tablets, USP are indicated: * For relief of signs and symptoms of juvenile arthritis * For relief of the signs and symptoms of rheumatoid arthritis * For relief of the signs and symptoms of osteoarthritis
Dosage and Administration
Dosage and Administration Carefully consider the potential benefits and risks of etodolac extended-release tablets and other treatment options before deciding to use etodolac extended-release tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS). After observing the response to initial therapy with etodolac extended-release tablets, the dose and frequency should be adjusted to suit an individual patient’s needs. Juvenile Rheumatoid Arthritis For the relief of the signs and symptoms of juvenile rheumatoid arthritis in patients 6 to 16 years of age, the recommended dose given orally once per day should be based on body weight, according to the following table: Table 4 Body Weight Range (kg) Dose 20 to 30 400 mg Tablet x 1 31 to 45 600 mg Tablet x 1 46 to 60 400 mg Tablet x 2 > 60 500 mg Tablet x 2 Rheumatoid Arthritis and Osteoarthritis For the relief of the signs and symptoms of osteoarthritis or rheumatoid arthritis, the recommended starting dose of etodolac extended-release tablets is 400 to 1000 mg given orally once per day. As with other NSAIDs, the lowest effective dose should be sought for each patient. In chronic conditions, a therapeutic response to therapy with etodolac extended-release tablets is sometimes seen within one week of therapy, but most often is observed by two weeks.
