Drug Catalog - Product Detail
ETODOLAC CAP 200 MG 100 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62559-0250-01 | ANI PHARMACEUTICALS | 100 | 200MG | CAPSULE |
PACKAGE FILES




Generic Name
ETODOLAC
Substance Name
ETODOLAC
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075126
Description
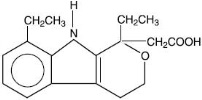
DESCRIPTION Etodolac is a member of the pyranocarboxylic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each capsule contains etodolac for oral administration. Etodolac is a racemic mixture of [+]S and [-]R-enantiomers. Etodolac is a white crystalline compound, insoluble in water but soluble in alcohols, chloroform, dimethyl sulfoxide, and aqueous polyethylene glycol. The chemical name is (±)1,8-diethyl-1,3,4,9-tetrahydropyrano-[3,4-b]indole-1-acetic acid. The molecular weight of the base is 287.37. It has a pKa of 4.65 and an n-octanol:water partition coefficient of 11.4 at pH 7.4. The molecular formula for etodolac is C 17 H 21 NO 3 , and it has the following structural formula: Each capsule, for oral administration, contains 200 mg or 300 mg of etodolac USP. The inactive ingredients in Etodolac Capsules USP include: lactose monohydrate, povidone, sodium starch glycolate, sodium lauryl sulfate, propylene glycol, colloidal silicon dioxide, magnesium stearate, talc, titanium dioxide, gelatin, D&C Red No. 28, D&C Red No. 33, FD&C Red No. 40, D&C Yellow No. 10, FD&C Blue No. 1, shellac, and black iron oxide. structure
How Supplied
HOW SUPPLIED Etodolac Capsules USP, are available as follows: 200 mg: hard gelatin capsules with an opaque pale red body and an opaque dark red cap, imprinted with “ANI” on the cap and “250” on the body in gray ink; available in bottles of 100 (NDC 62559-250-01). 300 mg: hard gelatin capsules with an opaque dark red body and cap, imprinted with “ANI” on the cap and “251” on the body in gray ink; available in bottles of 100 (NDC 62559-251-01). Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature], protected from moisture. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Keep container tightly closed. Manufactured by: ANI Pharmaceuticals, Inc. Baudette, MN 56623 9687 Rev 09/21 ani-logo
Indications & Usage
INDICATIONS AND USAGE Carefully consider the potential benefits and risks of etodolac capsules and other treatment options before deciding to use etodolac capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). Etodolac capsules are indicated: • For acute and long-term use in the management of signs and symptoms of the following: 1. Osteoarthritis 2. Rheumatoid arthritis • For the management of acute pain
Dosage and Administration
DOSAGE AND ADMINISTRATION Carefully consider the potential benefits and risks of etodolac capsules and other treatment options before deciding to use etodolac capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). After observing the response to initial therapy with etodolac capsules, the dose and frequency should be adjusted to suit an individual patient's needs. Dosage adjustment of etodolac capsules is generally not required in patients with mild to moderate renal impairment. Etodolac should be used with caution in such patients, because, as with other NSAIDs, it may further decrease renal function in some patients with impaired renal function (see WARNINGS, Renal Effects ). Analgesia The recommended total daily dose of etodolac capsules for acute pain is up to 1000 mg, given as 200 to 400 mg every 6 to 8 hours. Doses of etodolac greater than 1000 mg/day have not been adequately evaluated in well-controlled trials. Osteoarthritis and Rheumatoid Arthritis The recommended starting dose of etodolac capsules for the management of the signs and symptoms of osteoarthritis or rheumatoid arthritis is: 300 mg b.i.d., t.i.d., or 400 mg b.i.d., or 500 mg b.i.d. A lower dose of 600 mg/day may suffice for long-term administration. Physicians should be aware that doses above 1000 mg/day have not been adequately evaluated in well-controlled clinical trials. In chronic conditions, a therapeutic response to therapy with etodolac capsules is sometimes seen within one week of therapy, but most often is observed by two weeks. After a satisfactory response has been achieved, the patient's dose should be reviewed and adjusted as required.
