Drug Catalog - Product Detail
ETHOSUXIMIDE CAP 250 MG 100 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69452-0152-20 | BIONPHARMA | 100 | 250MG | CAPSULE |
PACKAGE FILES

Generic Name
ETHOSUXIMIDE
Substance Name
ETHOSUXIMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040430
Description
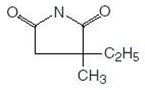
DESCRIPTION Ethosuximide is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula: Each ethosuximide capsule, USP contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol. The capsule contains FD&C yellow No. 6, gelatin, glycerin, hypromellose, iron oxide black, propylene glycol, and purified water. Chemical Structure
How Supplied
HOW SUPPLIED Ethosuximide capsules, USP 250 mg are supplied as: Clear, orange-colored, oval-shaped, softgel capsules in bottles of 100, Printed PA1000 NDC 69452-152-20. Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP. Protect from moisture.
Indications & Usage
INDICATIONS AND USAGE Ethosuximide capsules are indicated for the control of absence (petit mal) epilepsy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Ethosuximide capsules are administered by the oral route. The initial dose for patients 3 years to 6 years of age is one capsule (250 mg) per day; for patients 6 years of age and older, 2 capsules (500 mg) per day. The dose thereafter must be individualized according to the patient's response. Dosage should be increased by small increments. One useful method is to increase the daily dose by 250 mg every four to seven days until control is achieved with minimal side effects. Dosages exceeding 1.5 g daily, in divided doses, should be administered only under the strictest supervision of the physician. The optimal dose for most pediatric patients is 20 mg/kg/day. This dose has given average plasma levels within the accepted therapeutic range of 40 mcg/mL to 100 mcg/mL. Subsequent dose schedules can be based on effectiveness and plasma level determinations. Ethosuximide capsules may be administered in combination with other anticonvulsants when other forms of epilepsy coexist with absence (petit mal). The optimal dose for most pediatric patients is 20 mg/kg/day.
