Drug Catalog - Product Detail
ETHAMBUTOL USP TB 400MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 54879-0002-01 | STI PHARMA | 100 | 400MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
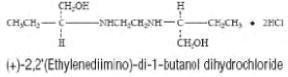
DESCRIPTION ETHAMBUTOL HYDROCHLORIDE is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium , including M. tuberculosis . The structural formula is: ETHAMBUTOL HYDROCHLORIDE (HCI) 100 and 400 mg tablets contain the following inactive ingredients: Gelatin, Hydroxypropyl Methylcellulose, Magnesium Stearate, Sodium Lauryl Sulfate, Sorbitol, Stearic Acid, Sucrose, Titanium Dioxide and other ingredients. structural formula
How Supplied
HOW SUPPLIED ETHAMBUTOL HYDROCHLORIDE TABLETS USP 100 mg – round, convex, white, film coated tablets engraved E6 on one side are supplied as follows: NDC 54879-001-00 - Carton of 10x10 Blister sheets & 54879-001-01 - Bottle of 100's 400 mg – round, convex, white, scored, film coated tablets engraved with E to the left and 7 to the right of the score on one side are supplied as follows: NDC 54879-002-00 - Carton of 10x10 Blister sheets & 54879-002-01 - Bottle of 100's Store at controlled room temperature 20 ° to 25 ° C (68 ° to 77 ° F). Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. Manufactured For & Distributed by: STI Pharma LLC. Langhorne, PA 19047 Iss. 08/12
Indications & Usage
INDICATIONS ETHAMBUTOL HCI is indicated for the treatment of pulmonary tuberculosis. It should not be used as the sole antituberculous drug, but should be used in conjunction with at least one other antituberculous drug. Selection of the companion drug should be based on clinical experience, considerations of comparative safety, and appropriate in vitro susceptibility studies. In patients who have not received previous antituberculous therapy, ie, initial treatment, the most frequently used regimens have been the following: ETHAMBUTOL HCI plus isoniazid ETHAMBUTOL HCI plus isoniazid plus streptomycin. In patients who have received previous antituberculous therapy, mycobacterial resistance to other drugs used in initial therapy is frequent. Consequently, in such retreatment patients, ETHAMBUTOL HCI should be combined with at least one of the second line drugs not previously administered to the patient and to which bacterial susceptibility has been indicated by appropriate in vitro studies. Antituberculous drugs used with ETHAMBUTOL HCI have included cycloserine, ethionamide, pyrazinamide, viomycin and other drugs. Isoniazid, aminosalicylic acid, and streptomycin have also been used in multiple drug regimens. Alternating drug regimens have also been utilized.
Dosage and Administration
DOSAGE and ADMINISTRATION ETHAMBUTOL HCI should not be used alone, in initial treatment or in tretreatment. ETHAMBUTOL HCI should be administered on a once every 24-hour basis only. Absorption is not significantly altered by administration with food. Therapy, in general, should be continued until bacteriological conversion has become permanent and maximal clinical improvement has occurred. ETHAMBUTOL HCI is not recommended for use in pediatric patients under thirteen years of age since safe conditions for use have not been established. Initial Treatment: In patients who have not received previous antituberculous therapy, administer ETHAMBUTOL HCI 15 mg/kg (7 mg/lb) of body weight, as a single oral dose once every 24 hours. In the more recent studies, isoniazid has been administered concurrently in a single, daily, oral dose. Retreatment: In patients who have received previous antituberculous therapy, administer ETHAMBUTOL HCI 25 mg/kg (11 mg/lb) of body weight, as a single oral dose once every 24 hours. Concurrently administer at least one other antituberculous drug to which the organisms have been demonstrated to be susceptible by appropriate in vitro tests. Suitable drugs usually consist of those not previously used in the treatment of the patient. After 60 days of ETHAMBUTOL HCI administration, decrease the dose to 15 mg/kg (7mg/lb) of body weight, and administer as a single oral dose once every 24 hours. During the period when a patient is on a daily dose of 25 mg/kg, monthly eye examinations are advised. See Table for easy selection of proper weight-dose tablet(s). Weight-Dose Table 15 mg/kg (7 mg/lb) Schedule Weight Range Pounds Kilograms Dose In mg Under 85 Under 37…………. ……………….500 85 – 94.5 37 – 43 ................. ……………….600 95 – 109.5 43 – 50 ................. ……………….700 110– 124.5 50 – 57 ................. ……………….800 125– 139.5 57 – 64 ................. ……………….900 140– 154.5 64 – 71 ................. ……………..1000 155– 169.5 71 – 79 ................. ……………..1100 170 – 184.5 79 – 84 ................. ……………..1200 185– 199.5 84 – 90 ................. ……………..1300 200– 214.5 90 – 97 ................. ……………..1400 215 and Over Over 97 ................ ……………..1500 25 mg/kg (11 mg/lb) Schedule Under 85 Under 38............... ……………..900 85 – 92.5 38 – 42................. ……………..1000 93 – 101.5 42 – 45.5............... ……………..1100 102– 109.5 45.5 – 50............... ……………..1200 110 – 118.5 50 – 54................. ……………..1300 119 – 128.5 54 – 58................. ……………..1400 129 – 136.5 58 – 62................. ……………..1500 137 – 146.5 62 – 67................. ……………..1600 147 – 155.5 67 – 71................. ……………..1700 156 – 164.5 71 – 75................. ……………..1800 165– 173.5 75 – 79................. ……………..1900 174– 182.5 79 – 83................. ……………..2000 183– 191.5 83 – 87................. ……………..2100 192– 199.5 87 – 91................. ……………..2200 200– 209.5 91 – 95................. ……………..2300 210– 218.5 95 – 99................. ……………..2400 219 and Over Over 99................. ……………..2500
