Drug Catalog - Product Detail
ESTRADIOL VAGINAL CREAM 0.01% 42.5GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-8770-35 | MYLAN | 42 | 0.1MG/GM | CREAM |
PACKAGE FILES






Generic Name
ESTRADIOL
Substance Name
ESTRADIOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
VAGINAL
Application Number
ANDA208788
Description
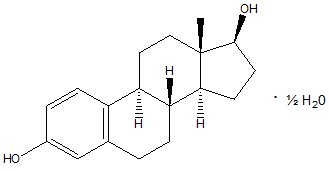
DESCRIPTION Each gram of estradiol vaginal cream USP, 0.01% contains estradiol, USP hemihydrate equivalent to 0.1 mg estradiol in a nonliquefying base containing edetate disodium, hypromellose, methylparaben, mono- and di-glycerides, propylene glycol, purified water, sodium lauryl sulfate, stearyl alcohol, tert -butylhydroquinone and white ceresin wax. Estradiol is chemically described as Estra-1,3,5(10)-triene-3,17β-diol-hemihydrate. It has an empirical formula of C 18 H 24 O 2 • ½ H 2 O and molecular weight of 281.39. The structural formula is: Estradiol Structural Formula
How Supplied
HOW SUPPLIED Estradiol Vaginal Cream USP, 0.01% contains estradiol, USP hemihydrate equivalent to 0.1 mg estradiol in a nonliquefying base. The opaque, white to off white cream is available in a tube containing 1.5 oz (42.5 g) with a calibrated plastic applicator for delivery of 1 g, 2 g, 3 g, or 4 g. It is available as follows: NDC 0378-8770-35 carton containing one 1.5 oz (42.5 g) tube Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from temperatures in excess of 40°C (104°F). Keep estradiol vaginal cream 0.01% out of the reach of children. PHARMACIST: Dispense the Patient Information Leaflet with each prescription.
Indications & Usage
INDICATIONS AND USAGE Estradiol vaginal cream 0.01% is indicated in the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause.
Dosage and Administration
DOSAGE AND ADMINISTRATION Use of estradiol vaginal cream 0.01% alone or in combination with a progestin, should be limited to the shortest duration consistent with treatment goals and risks for the individual woman. Postmenopausal women should reevaluate periodically as clinically appropriate to determine if treatment is still necessary. For treatment of vulvar and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible. For women who have a uterus, adequate diagnostic measures, including directed and random endometrial sampling when indicated, should be undertaken to rule out malignancy in cases of undiagnosed persistent or recurring abnormal genital bleeding. Usual Dosage: The usual dosage range is 2 to 4 g (marked on the applicator) daily for one or two weeks, then gradually reduced to one half initial dosage for a similar period. A maintenance dosage of 1 g, one to three times a week, may be used after restoration of the vaginal mucosa has been achieved. NOTE: The number of doses per tube will vary with dosage requirements and patient handling.
