Drug Catalog - Product Detail
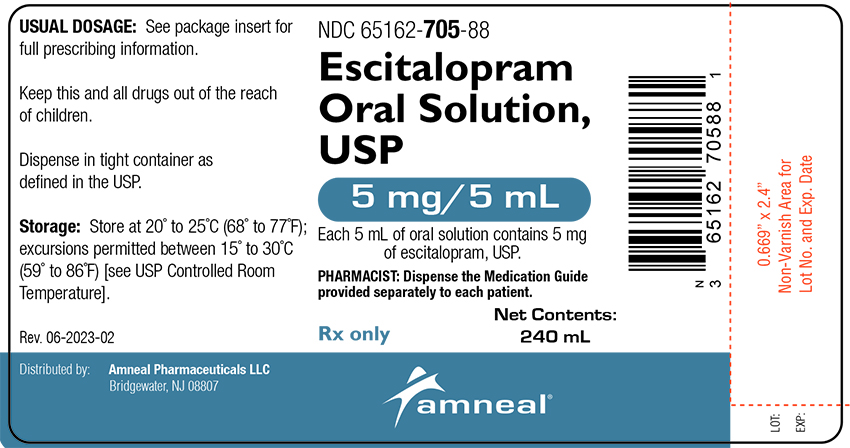
ESCITALOPRAM OXALATE ORAL SOLUTION SOL 5MG/5ML 240ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0705-88 | AMNEAL PHARMACEUTICALS | 240 | 5MG/5ML | SOLUTION |
PACKAGE FILES
Generic Name
ESCITALOPRAM
Substance Name
ESCITALOPRAM OXALATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202227
Description
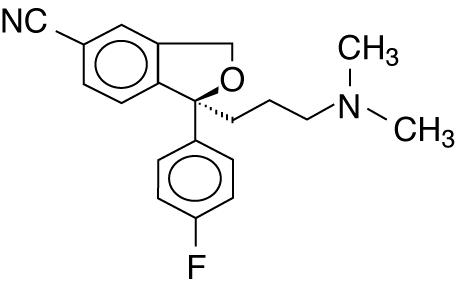
11 DESCRIPTION Escitalopram oral solution, USP contains escitalopram oxalate USP, a selective serotonin reuptake inhibitor (SSRI), present as escitalopram oxalate salt. Escitalopram is the pure S-enantiomer (single isomer) of the racemic bicyclic phthalane derivative citalopram. Escitalopram oxalate, USP is designated S-(+)-1-[3-(dimethyl-amino)propyl]-1-(p - fluorophenyl)-5-phthalancarbonitrile oxalate with the following structural formula: •C 2 H 2 O 4 The molecular formula is C 20 H 21 FN 2 O • C 2 H 2 O 4 and the molecular weight is 414.40. Escitalopram oxalate, USP occurs as a fine, white to slightly-yellow powder and is freely soluble in methanol and dimethyl sulfoxide (DMSO), soluble in isotonic saline solution, sparingly soluble in water and ethanol, slightly soluble in ethyl acetate, and insoluble in heptane. Escitalopram oral solution, USP contains 1.29 mg/mL escitalopram oxalate, USP equivalent to 1 mg/mL escitalopram base. It also contains the following inactive ingredients: anhydrous citric acid, glycerin, malic acid, methylparaben, natural peppermint flavor, propylene glycol, propylparaben, purified water, sodium citrate and sorbitol. ec45d723-figure-01
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Oral Solution Escitalopram oral solution, USP, equivalent 5 mg base/5 mL , is a clear colorless to pale yellow, peppermint flavored solution, available as 240 mL; NDC 65162-705-88. Storage and Handling Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Escitalopram oral solution is indicated for the treatment of: major depressive disorder (MDD) in adults and pediatric patients 12 years of age and older. generalized anxiety disorder (GAD) in adults. Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. Escitalopram oral solution is a selective serotonin reuptake inhibitor (SSRI) indicated for the: treatment of major depressive disorder (MDD) in adults and pediatric patients 12 years of age and older. ( 1 ) treatment of generalized anxiety disorder (GAD) in adults. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Indication and Population Recommended Dosage MDD in Adults (2.1) Initial: 10 mg once daily Recommended: 10 mg once daily Maximum: 20 mg once daily MDD in Pediatric Patients 12 years and older (2.1) Initial: 10 mg once daily Recommended: 10 mg once daily Maximum: 20 mg once daily GAD in Adults (2.2) Initial: 10 mg once daily Recommended: 10 mg once daily Maximum: 20 mg once daily No additional benefits were seen at 20 mg once daily. (2.1) Administer once daily, morning or evening, with or without food. (2.3) Elderly patients: recommended dosage is 10 mg once daily. (2.4) Hepatic impairment: recommended dosage is 10 mg once daily. (2.4 , 8.6 ) When discontinuing escitalopram oral solution, reduce dose gradually whenever possible. (2.5) 2.1 Major Depressive Disorder Adults The recommended dosage of escitalopram oral solution in adults is 10 mg once daily. A fixed-dose trial of escitalopram oral solution demonstrated the effectiveness of both 10 mg and 20 mg of escitalopram oral solution, but failed to demonstrate a greater benefit of 20 mg over 10 mg [see Clinical Studies (14.1) ] . Depending on clinical response and tolerability, dosage may be increased to the maximum recommended dosage of 20 mg once daily at an interval of no less than 1 week. Pediatric Patients 12 years of age and older The recommended dosage of escitalopram oral solution in pediatric patients 12 years of age and older is 10 mg once daily. Depending on clinical response and tolerability, dosage may be increased to the maximum recommended dosage of 20 mg once daily at an interval of no less than 3 weeks. 2.2 Generalized Anxiety Disorder Adults The recommended starting dosage of escitalopram oral solution in adults is 10 mg once daily. Depending on clinical response and tolerability, dosage may be increased to the maximum recommended dosage of 20 mg once daily at an interval of no less than 1 week. Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. 2.3 Administration Information Administer escitalopram oral solution orally once daily, in the morning or evening, with or without food. 2.4 Screen for Bipolar Disorder Prior to Starting Escitalopram Oral Solution Prior to initiating treatment with escitalopram oral solution or another antidepressant, screen patients for a personal family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.5) ] . 2.5 Recommended Dosage for Specific Populations The recommended dosage for most elderly patients and patients with hepatic impairment is 10 mg once daily [see Use in Specific Populations (8.5 , 8.6) ] . The recommended dosage for escitalopram oral solution in adults with a creatinine clearance less than 20 mL/minute has not been determined. No dosage adjustment is necessary for patients with mild or moderate renal impairment [see Use in Specific Populations (8.7) ] . 2.6 Discontinuation of Treatment with Escitalopram Oral Solution Symptoms associated with discontinuation of escitalopram oral solution and other SSRIs and SNRIs have been reported [see Warnings and Precautions (5.3) ] . Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate. 2.7 Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with escitalopram oral solution. Conversely, at least 14 days should be allowed after stopping escitalopram oral solution before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4) ] .
