Drug Catalog - Product Detail
ENALAPRIL MALEATE 2.5MG TB 1000CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68682-0710-10 | OCEANSIDE PHARMACEUTICALS | 1000 | 2.5MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
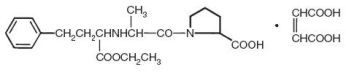
DESCRIPTION Enalapril maleate is the maleate salt of enalapril, the ethyl ester of a long-acting angiotensin converting enzyme inhibitor, enalaprilat. Enalapril maleate is chemically described as (S)-1-[ N -[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline, ( Z )-2-butenedioate salt (1:1). Its empirical formula is C 20 H 28 N 2 O 5 •C 4 H 4 O 4 , and its structural formula is: Enalapril maleate is a white to off-white, crystalline powder with a molecular weight of 492.53. It is sparingly soluble in water, soluble in ethanol, and freely soluble in methanol. Enalapril is a pro-drug; following oral administration, it is bioactivated by hydrolysis of the ethyl ester to enalaprilat, which is the active angiotensin converting enzyme inhibitor. Enalapril maleate is supplied as 2.5 mg, 5 mg, 10 mg, and 20 mg tablets for oral administration. In addition to the active ingredient enalapril maleate, each tablet contains the following inactive ingredients: lactose, magnesium stearate, sodium1 bicarbonate and starch. The 10 mg and 20 mg tablets also contain iron oxides. image
How Supplied
HOW SUPPLIED Enalapril maleate tablets, 2.5 mg, are white, oval shaped tablet with “VASO 2.5” and scored on one side and scored on the other. They are supplied as follows: 68682-710-30 bottles of 30 (with desiccant) 68682-710-90 unit of use bottles of 90 (with desiccant) 68682-710-01 bottles of 100 (with desiccant) 68682-710-10 bottles of 1,000 (with desiccant) Enalapril maleate tablets, 5 mg, are white, rounded triangle shaped tablet with “VASO 5” on one side and scored on the other. They are supplied as follows: 68682-711-30 bottles of 30 (with desiccant) 68682-711-90 unit of use bottles of 90 (with desiccant) 68682-711-01 bottles of 100 (with desiccant) 68682-711-10 bottles of 1,000 (with desiccant) Enalapril maleate tablets, 10 mg, are rust red, rounded triangle shaped tablet with “VASO 10” on one side and scored on the other. They are supplied as follows: 68682-712-30 bottles of 30 (with desiccant) 68682-712-90 unit of use bottles of 90 (with desiccant) 68682-712-01 bottles of 100 (with desiccant) 68682-712-10 bottles of 1,000 (with desiccant) Enalapril maleate tablets, 20 mg, are peach, rounded triangle shaped tablet with “VASO 20” on one side and scored on the other. They are supplied as follows: 68682-713-30 bottles of 30 (with desiccant) 68682-713-90 unit of use bottles of 90 (with desiccant) 68682-713-01 bottles of 100 (with desiccant) 68682-713-10 bottles of 1,000 (with desiccant) Storage Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from moisture. Dispense in a tight container as per USP, if product package is subdivided. Vasotec ® is a registered trademark of Valeant Pharmaceuticals North America LLC. Manufactured for: Oceanside Pharmaceuticals, a division of Valeant Pharmaceuticals North America LLC Bridgewater, NJ 08807 USA By: Valeant Pharmaceuticals International, Inc. Steinbach, MB R5G 1Z7, Canada Made in Canada 20000940 Rev. 05/15 9450101
Indications & Usage
INDICATIONS AND USAGE Hypertension Enalapril maleate tablet is indicated for the treatment of hypertension. Enalapril maleate tablet is effective alone or in combination with other antihypertensive agents, especially thiazide-type diuretics. The blood pressure lowering effects of enalapril maleate tablets and thiazides are approximately additive. Heart Failure Enalapril maleate tablet is indicated for the treatment of symptomatic congestive heart failure, usually in combination with diuretics and digitalis. In these patients enalapril maleate tablet improves symptoms, increases survival, and decreases the frequency of hospitalization (see CLINICAL PHARMACOLOGY , Heart Failure, Mortality Trials for details and limitations of survival trials). Asymptomatic Left Ventricular Dysfunction In clinically stable asymptomatic patients with left ventricular dysfunction (ejection fraction ≤35 percent), enalapril maleate tablet decreases the rate of development of overt heart failure and decreases the incidence of hospitalization for heart failure (see CLINICAL PHARMACOLOGY , Heart Failure, Mortality Trials for details and limitations of survival trials). In using enalapril maleate tablets consideration should be given to the fact that another angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that enalapril maleate tablet does not have a similar risk (see WARNINGS ). In considering use of enalapril maleate tablets, it should be noted that in controlled clinical trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, it should be noted that black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non-blacks (see WARNINGS , Head and Neck Angioedema ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Hypertension In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally may occur following the initial dose of enalapril maleate tablets. The diuretic should, if possible, be discontinued for two to three days before beginning therapy with enalapril maleate tablets to reduce the likelihood of hypotension (see WARNINGS ). If the patient’s blood pressure is not controlled with enalapril maleate tablets alone, diuretic therapy may be resumed. If the diuretic cannot be discontinued an initial dose of 2.5 mg should be used under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour (see WARNINGS and PRECAUTIONS , Drug Interactions ). The recommended initial dose in patients not on diuretics is 5 mg once a day. Dosage should be adjusted according to blood pressure response. The usual dosage range is 10 to 40 mg per day administered in a single dose or two divided doses. In some patients treated once daily, the antihypertensive effect may diminish toward the end of the dosing interval. In such patients, an increase in dosage or twice daily administration should be considered. If blood pressure is not controlled with enalapril maleate tablets alone, a diuretic may be added. Concomitant administration of enalapril maleate tablets with potassium supplements, potassium salt substitutes, or potassium-sparing diuretics may lead to increases of serum potassium (see PRECAUTIONS ). Dosage Adjustment in Hypertensive Patients with Renal Impairment The usual dose of enalapril is recommended for patients with a creatinine clearance >30 mL/min (serum creatinine of up to approximately 3 mg/dL). For patients with creatinine clearance ≤30 mL/min (serum creatinine ≥3 mg/dL), the first dose is 2.5 mg once daily. The dosage may be titrated upward until blood pressure is controlled or to a maximum of 40 mg daily. Renal Status Creatinine- Clearance mL/min Initial Dose mg/day Normal Renal Function >80 mL/min 5 mg Mild Impairment ≤80 >30 mL/min 5 mg Moderate to Severe Impairment ≤30 mL/min 2.5 mg Dialysis Patients* - 2.5 mg on dialysis days † * See WARNINGS , Anaphylactoid reactions during membrane exposure . † Dosage on nondialysis days should be adjusted depending on the blood pressure response. Heart Failure Enalapril maleate tablet is indicated for the treatment of symptomatic heart failure, usually in combination with diuretics and digitalis. In the placebo-controlled studies that demonstrated improved survival, patients were titrated as tolerated up to 40 mg, administered in two divided doses. The recommended initial dose is 2.5 mg. The recommended dosing range is 2.5 to 20 mg given twice a day. Doses should be titrated upward, as tolerated, over a period of a few days or weeks. The maximum daily dose administered in clinical trials was 40 mg in divided doses. After the initial dose of enalapril maleate tablets, the patient should be observed under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour (see WARNINGS and PRECAUTIONS , Drug Interactions ). If possible, the dose of any concomitant diuretic should be reduced which may diminish the likelihood of hypotension. The appearance of hypotension after the initial dose of enalapril maleate tablets does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension. Asymptomatic Left Ventricular Dysfunction In the trial that demonstrated efficacy, patients were started on 2.5 mg twice daily and were titrated as tolerated to the targeted daily dose of 20 mg (in divided doses). After the initial dose of enalapril maleate tablets, the patient should be observed under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour (see WARNINGS and PRECAUTIONS , Drug Interactions ). If possible, the dose of any concomitant diuretic should be reduced which may diminish the likelihood of hypotension. The appearance of hypotension after the initial dose of enalapril maleate tablets does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension. Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia In patients with heart failure who have hyponatremia (serum sodium less than 130 mEq/L) or with serum creatinine greater than 1.6 mg/dL, therapy should be initiated at 2.5 mg daily under close medical supervision (see DOSAGE AND ADMINISTRATION , Heart Failure, WARNINGS and PRECAUTIONS , Drug Interactions ). The dose may be increased to 2.5 mg b.i.d., then 5 mg b.i.d. and higher as needed, usually at intervals of four days or more if at the time of dosage adjustment there is not excessive hypotension or significant deterioration of renal function. The maximum daily dose is 40 mg. Pediatric Hypertensive Patients The usual recommended starting dose is 0.08 mg/kg (up to 5 mg) once daily. Dosage should be adjusted according to blood pressure response. Doses above 0.58 mg/kg (or in excess of 40 mg) have not been studied in pediatric patients (see CLINICAL PHARMACOLOGY , Clinical Pharmacology in Pediatric Patients ). Enalapril maleate tablet is not recommended in neonates and in pediatric patients with glomerular filtration rate <30 mL/min/1.73 m 2 , as no data are available. Preparation of Suspension (for 200 mL of a 1.0 mg/mL suspension) Add 50 mL of Bicitra ®1 to a polyethylene terephthalate (PET) bottle containing ten 20 mg tablets of enalapril maleate and shake for at least 2 minutes. Let concentrate stand for 60 minutes. Following the 60-minute hold time, shake the concentrate for an additional minute. Add 150 mL of Ora-Sweet SF TM2 to the concentrate in the PET bottle and shake the suspension to disperse the ingredients. The suspension should be refrigerated at 2-8°C (36-46°F) and can be stored for up to 30 days. Shake the suspension before each use. 1 Registered trademark of Alza Corporation 2 Trademark of Paddock Laboratories, Inc.
