Drug Catalog - Product Detail
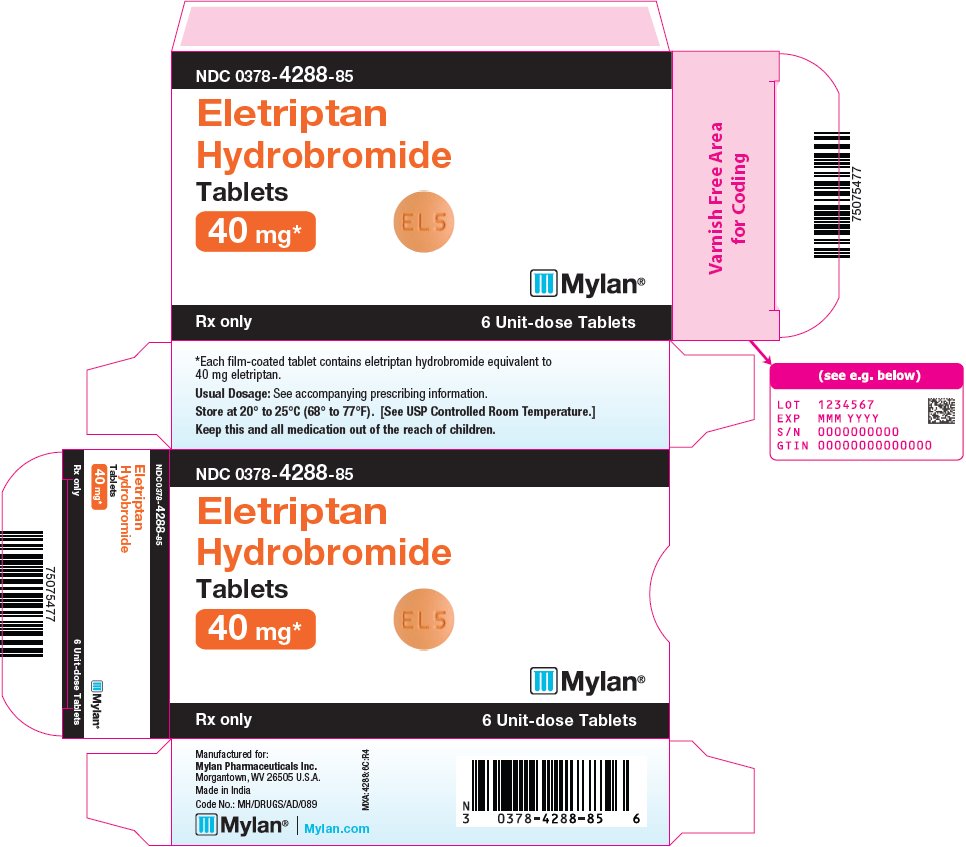
ELETRIPTAN HYDROBROMIDE 40MG TABS 1X6 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-4288-85 | MYLAN | 6 | 40MG | NA |
PACKAGE FILES
Generic Name
ELETRIPTAN HYDROBROMIDE
Substance Name
ELETRIPTAN HYDROBROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA205152
Description
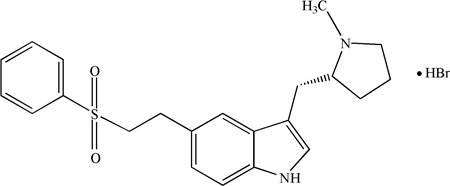
11 DESCRIPTION Eletriptan hydrobromide tablets contain eletriptan hydrobromide, which is a selective 5-hydroxytryptamine 1B/1D (5-HT 1B/1D ) receptor agonist. Eletriptan hydrobromide is chemically designated as (R)-3-[(1-Methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-indole monohydrobromide, and it has the following chemical structure: The molecular formula is C 22 H 26 N 2 O 2 S • HBr, representing a molecular weight of 463.43. Eletriptan hydrobromide is an off-white to light brown colored powder that is readily soluble in water. Each eletriptan hydrobromide tablet for oral administration contains 24.231 or 48.462 mg of eletriptan hydrobromide equivalent to 20 mg or 40 mg of eletriptan, respectively. Each tablet also contains the inactive ingredients anhydrous lactose, croscarmellose sodium, FD&C Yellow No. 6 Aluminum Lake, lecithin, microcrystalline cellulose, magnesium stearate, polyvinyl alcohol, talc, titanium dioxide and xanthan gum. Eletriptan Hydrobromide Structural Formula
How Supplied
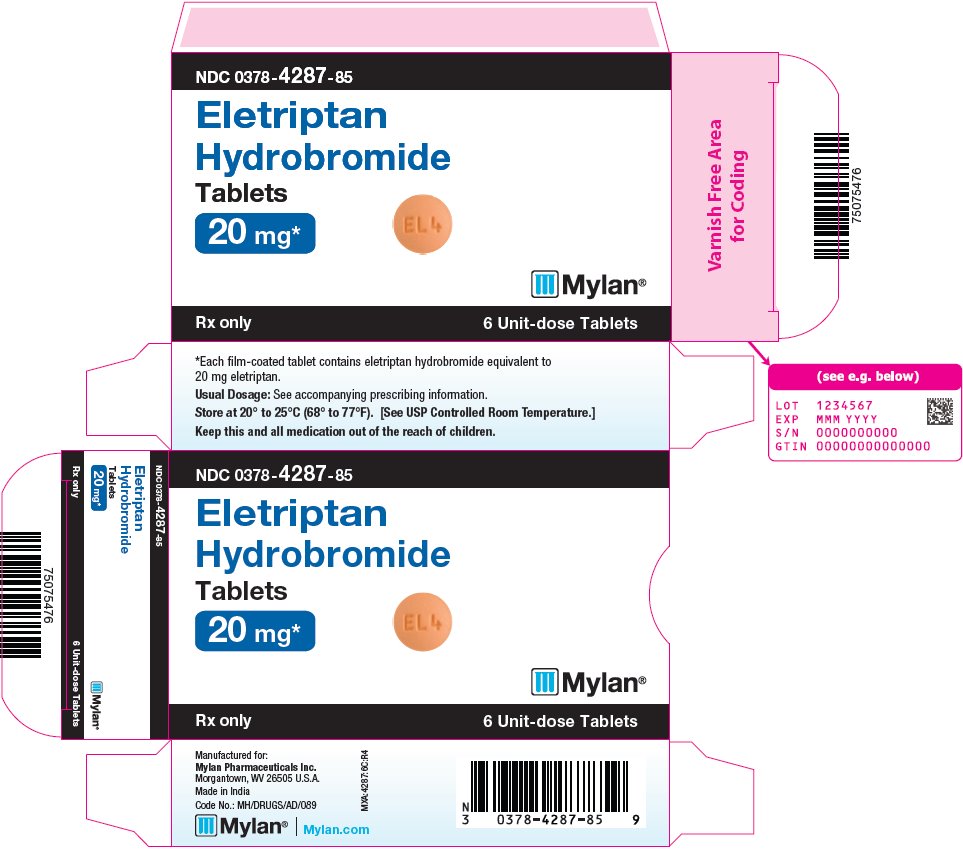
16 HOW SUPPLIED/STORAGE AND HANDLING Eletriptan Hydrobromide Tablets are available containing eletriptan hydrobromide equivalent to 20 mg or 40 mg eletriptan (base), respectively. The 20 mg tablets are orange, film-coated, round, unscored tablets debossed with M on one side of the tablet and EL4 on the other side. They are available as follows: NDC 0378-4287-85 carton of 6 unit-dose tablets (1 x 6) The 40 mg tablets are orange, film-coated, round, unscored tablets debossed with M on one side of the tablet and EL5 on the other side. They are available as follows: NDC 0378-4288-85 carton of 6 unit-dose tablets (1 x 6) NDC 0378-4288-08 carton of 12 unit-dose tablets (2 x 6) Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Indications & Usage
1 INDICATIONS AND USAGE Eletriptan hydrobromide tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: • Use only if a clear diagnosis of migraine has been established. If a patient has no response to the first migraine attack treated with eletriptan hydrobromide tablets, reconsider the diagnosis of migraine before eletriptan hydrobromide tablets are administered to treat any subsequent attacks. • Eletriptan hydrobromide tablets are not intended for the prevention of migraine attacks. • Safety and effectiveness of eletriptan hydrobromide tablets have not been established for cluster headache. Eletriptan hydrobromide tablets are a serotonin (5-HT 1B/1D ) receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults ( 1 ) Limitations of Use: • Use only after a clear diagnosis of migraine has been established ( 1 ) • Not indicated for the prophylactic therapy of migraine ( 1 ) • Not indicated for the treatment of cluster headache ( 1 )
Dosage and Administration
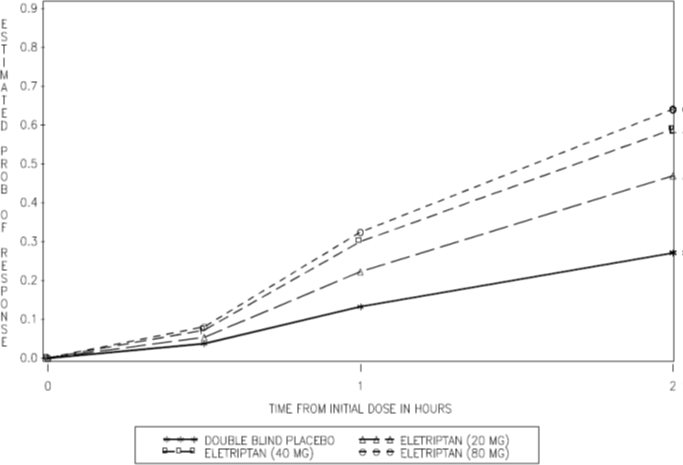
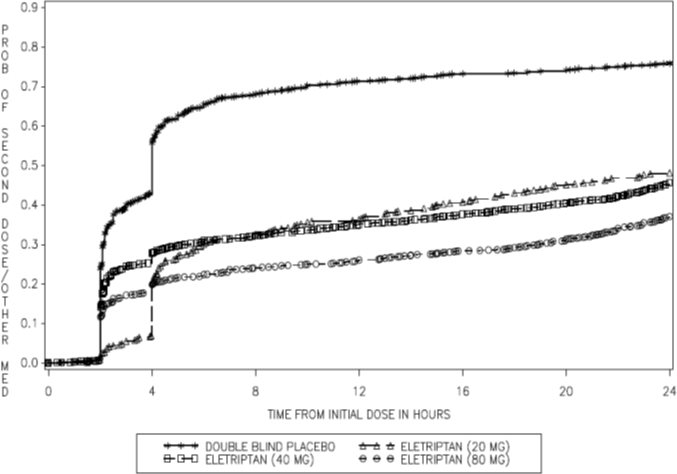
2 DOSAGE AND ADMINISTRATION The maximum recommended single dose is 40 mg. In controlled clinical trials, single doses of 20 mg and 40 mg were effective for the acute treatment of migraine in adults. A greater proportion of patients had a response following a 40 mg dose than following a 20 mg dose [see Clinical Studies (14) ] . If the migraine has not resolved by 2 hours after taking eletriptan hydrobromide tablets, or returns after transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose should not exceed 80 mg. The safety of treating an average of more than 3 migraine attacks in a 30-day period has not been established. • Single dose: 20 mg or 40 mg ( 2 ) • Maximum single dose: 40 mg ( 2 ) • May repeat dose after 2 hours if needed; not to exceed 80 mg in any 24-hour period ( 2 )
