Drug Catalog - Product Detail
EFAVIRENZ USP TABS 600MG 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0301-02 | CIPLA USA | 30 | 600MG | TABLET |
PACKAGE FILES

Generic Name
EFAVIRENZ
Substance Name
EFAVIRENZ
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204766
Description
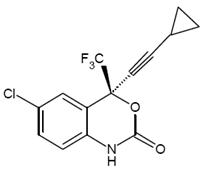
11 DESCRIPTION Efavirenz is an HIV-1 specific, non-nucleoside, reverse transcriptase inhibitor (NNRTI). Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its empirical formula is C 14 H 9 ClF 3 NO 2 and its structural formula is: Efavirenz is a white to slightly pink crystalline powder with a molecular mass of 315.68. It is practically insoluble in water (<10 microgram/mL). Tablets: Efavirenz tablets, USP is available as film-coated tablets for oral administration containing 600 mg of efavirenz and the following inactive ingredients: Lactose monohydrate, croscarmellose sodium, povidone, anhydrous dibasic calcium phosphate, magnesium stearate. The film coating contains opadry yellow (consist of hypromellose, titanium dioxide, macrogol, iron oxide yellow). Efavirenz
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.2 Tablets Efavirenz tablets, USP are available as follows: 600-mg tablets are yellow coloured, capsule-shaped, biconvex, film coated tablets debossed with '301' on one side and 'CL' on other side. Bottle of 30 tablets NDC 69097-301-02 16.3 Storage Efavirenz tablets, USP should be stored at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Efavirenz tablets are a non-nucleoside reverse transcriptase inhibitor indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 infection in adults and in pediatric patients at least 3 months old and weighing at least 3.5kg. ( 1 ) Efavirenz in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and in pediatric patients at least 3 months old and weighing at least 3.5 kg.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Efavirenz should be taken orally once daily on an empty stomach, preferably at bedtime. ( 2 ) Recommended adult dose: 600 mg. ( 2.2 ) With voriconazole, increase voriconazole maintenance dose to 400 mg every 12 hours and decrease efavirenz dose to 300 mg once daily using the capsule formulation. ( 2.2 ) With rifampin, increase efavirenz dose to 800 mg once daily for patients weighing 50 kg or more. ( 2.2 ) Pediatric dosing is based on weight. ( 2.3 ) 2.1 Hepatic Function Monitor hepatic function prior to and during treatment with efavirenz tablets [see Warnings and Precautions ( 5.9 )]. Efavirenz tablets are not recommended in patients with moderate or severe hepatic impairment (Child Pugh B or C) [see Warnings and Precautions ( 5.9 ) and Use in Specific Populations ( 8.6 )] . 2.2 Adults The recommended dosage of efavirenz is 600 mg orally, once daily, in combination with a protease inhibitor and/or nucleoside analogue reverse transcriptase inhibitors (NRTIs). It is recommended that efavirenz be taken on an empty stomach, preferably at bedtime. The increased efavirenz concentrations observed following administration of efavirenz with food may lead to an increase in frequency of adverse reactions [see Clinical Pharmacology ( 12.3 ) ] . Dosing at bedtime may improve the tolerability of nervous system symptoms [see Warnings and Precautions ( 5.6 ) , Adverse Reactions ( 6.1 ) , and Patient Counseling Information ( 17 )]. Efavirenz tablets should be swallowed intact with liquid. Concomitant Antiretroviral Therapy Efavirenz must be given in combination with other antiretroviral medications [see Indications and Usage ( 1 ) , Warnings and Precautions ( 5.3 ) , Drug Interactions ( 7.1 ) , and Clinical Pharmacology ( 12.3 ) ] . Dosage Adjustment If efavirenz is coadministered with voriconazole, the voriconazole maintenance dose should be increased to 400 mg every 12 hours and the efavirenz dose should be decreased to 300 mg once daily using the capsule formulation. Efavirenz tablets must not be broken [see Drug Interactions ( 7.1 , Table 5 ) and Clinical Pharmacology ( 12.3 , Tables 7 and 8 )] . If efavirenz is coadministered with rifampin to patients weighing 50 kg or more, an increase in the dose of efavirenz to 800 mg once daily is recommended [see Drug Interactions ( 7.1 , Table 5 ) and Clinical Pharmacology ( 12.3 , Table 8 )]. 2.3 Pediatric Patients It is recommended that efavirenz be taken on an empty stomach, preferably at bedtime. Table 1 describes the recommended dose of efavirenz for pediatric patients weighing at least 40 kg [see Clinical Pharmacology ( 12.3 ) ] . The recommended dosage of efavirenz for pediatric patients weighing 40 kg or greater is 600 mg once daily. Table 1: Efavirenz Dosing in Pediatric Patients b Tablets must not be crushed. Patient Body Weight Efavirenz Daily Dose Number of Tablets b and Strength to Administer at least 40 kg 600 mg one 600 mg tablet
