Drug Catalog - Product Detail
DUTASTERIDE CP 0.5MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1438-10 | AMNEAL PHARMACEUTICALS | 90 | 0.5MG | CAPSULE |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
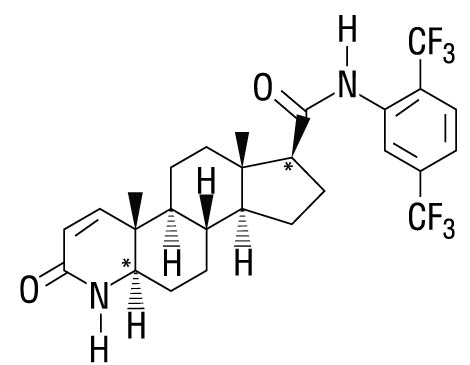
11 DESCRIPTION Dutasteride is a synthetic 4-azasteroid compound that is a selective inhibitor of both the type 1 and type 2 isoforms of steroid 5 alpha-reductase, an intracellular enzyme that converts testosterone to DHT. Dutasteride is chemically designated as (5a,17b)-N-{2,5 bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-1-ene-17-carboxamide. The empirical formula of dutasteride is C 27 H 30 F 6 N 2 O 2 , representing a molecular weight of 528.5 with the following structural formula: Dutasteride is a white to pale yellow powder with a melting point of 242° to 250°C. It is soluble in ethanol (44 mg/mL), methanol (64 mg/mL), and polyethylene glycol 400 (3 mg/mL), but it is insoluble in water. Each dutasteride capsule, administered orally, contains 0.5 mg of dutasteride dissolved in a mixture of mono-di-glycerides of caprylic/capric acid and butylated hydroxytoluene. The inactive excipients in the capsule shell are ferric oxide (yellow), gelatin (from certified BSE-free bovine sources), glycerin, and titanium dioxide. The capsules are printed with black ink (purified water, black iron oxide, isopropyl alcohol, propylene glycol, and Hypromellose). Dutasterided Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Dutasteride capsules 0.5 mg are oblong, opaque, dull yellow, gelatin capsules imprinted with M05 with black ink on one side packaged in bottles of 30 (NDC 0115-1438-08) and 90 (NDC 0115-1438-10) with child-resistant closures. Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Dutasteride is absorbed through the skin. Dutasteride capsules should not be handled by women who are pregnant or who may become pregnant because of the potential for absorption of dutasteride and the subsequent potential risk to a developing male fetus [see Warnings and Precautions ( 5.4 )] .
Indications & Usage
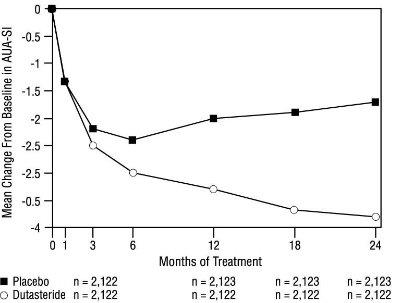
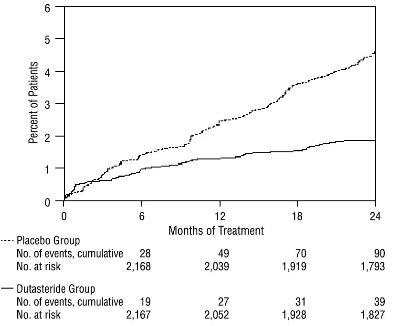
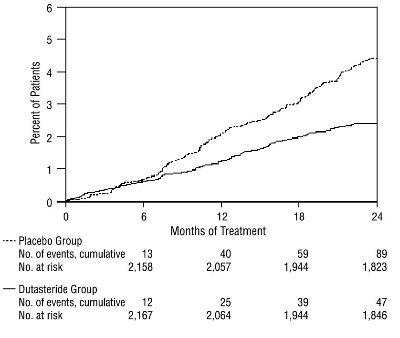
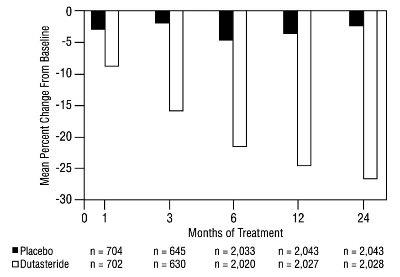
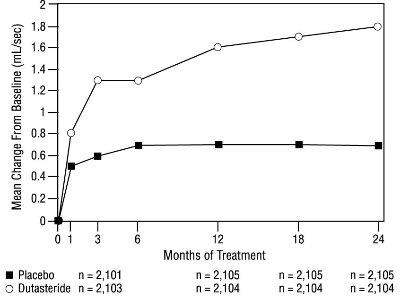
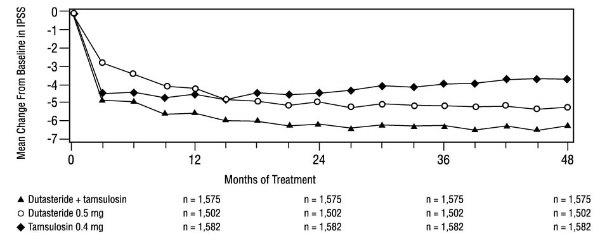
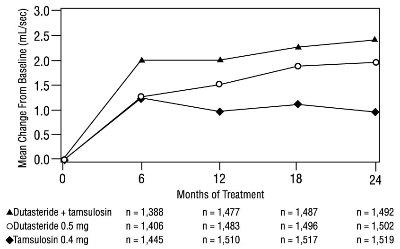
1 INDICATIONS AND USAGE Dutasteride is a 5 alpha-reductase inhibitor indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to: ( 1.1 ) improve symptoms, reduce the risk of acute urinary retention, and reduce the risk of the need for BPH-related surgery. Dutasteride in combination with the alpha adrenergic antagonist, tamsulosin, is indicated for the treatment of symptomatic BPH in men with an enlarged prostate. ( 1.2 ) Limitations of Use: Dutasteride is not approved for the prevention of prostate cancer. ( 1.3 ) 1.1 Monotherapy Dutasteride capsules are indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to: improve symptoms, reduce the risk of acute urinary retention (AUR), and reduce the risk of the need for BPH-related surgery. 1.2 Combination With Alpha Adrenergic Antagonist Dutasteride in combination with the alpha adrenergic antagonist, tamsulosin, is indicated for the treatment of symptomatic BPH in men with an enlarged prostate. 1.3 Limitations of Use Dutasteride is not approved for the prevention of prostate cancer.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The capsules should be swallowed whole and not chewed or opened, as contact with the capsule contents may result in irritation of the oropharyngeal mucosa. Dutasteride may be administered with or without food. Monotherapy: 0.5 mg once daily. ( 2.1 ) Combination with tamsulosin: 0.5 mg once daily and tamsulosin 0.4 mg once daily. ( 2.2 ) Dosing considerations: Swallow whole. May take with or without food. ( 2 ) 2.1 Monotherapy The recommended dose of dutasteride is 1 capsule (0.5 mg) taken once daily. 2.2 Combination With Alpha Adrenergic Antagonist The recommended dose of dutasteride is 1 capsule (0.5 mg) taken once daily and tamsulosin 0.4 mg taken once daily.
