Drug Catalog - Product Detail
DOXYCYCLINE MONOHYDRATE CAP. CP 150MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1327-13 | AMNEAL PHARMACEUTICALS | 60 | 150MG | CAPSULE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
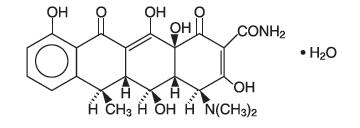
DESCRIPTION Doxycycline is a broad-spectrum antibacterial synthetically derived from oxytetracycline. Doxycycline capsules USP, 150 mg contain doxycycline monohydrate equivalent to 150 mg of doxycycline for oral administration. The chemical designation of the light-yellow crystalline powder is alpha-6-deoxy-5-oxytetracycline. Structural formula: C 22 H 24 N 2 O 8 ∙ H 2 O M.W. = 462.45 Doxycycline has a high degree of lipid solubility and a low affinity for calcium binding. It is highly stable in normal human serum. Doxycycline will not degrade into an epianhydro form. The inactive ingredients in doxycycline capsules, USP include colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The capsule shells contain gelatin, titanium dioxide, and FD&C Yellow No. 6. Additionally, the capsule imprint ink contains shellac, ferrosoferric oxide, propylene glycol, FD&C Blue No. 2, FD&C Red No. 40, D&C Yellow No. 10 Aluminum Lake, and FD&C Blue No. 1. Chemical Structure
How Supplied
HOW SUPPLIED Doxycycline Capsules USP, 150 mg have an orange opaque cap printed “M1” in black ink/orange opaque body. Each capsule contains doxycycline monohydrate equivalent to 150 mg of doxycycline. They are supplied as follows: Bottles of 30: NDC 0115-1327-08 Bottles of 60: NDC 0115-1327-13 Bottles of 90: NDC 0115-1327-10 Bottles of 100: NDC 0115-1327-01 Bottles of 1000: NDC 0115-1327-03 STORE AT 20° TO 25°C (68° TO 77°F) [SEE USP CONTROLLED ROOM TEMPERATURE]. DISPENSE IN A TIGHT LIGHT-RESISTANT CONTAINER AS DEFINED IN THE USP / NF.
Indications & Usage
INDICATIONS AND USAGE To reduce the development of drug-resistant bacteria and maintain effectiveness of doxycycline capsules, USP and other antibacterial drugs, doxycycline capsules, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Doxycycline is indicated for the treatment of the following infections: Rocky mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by Rickettsiae . Respiratory tract infections caused by Mycoplasma pneumoniae . Lymphogranuloma venereum caused by Chlamydia trachomatis . Psittacosis (ornithosis) caused by Chlamydophila psittaci . Trachoma caused by Chlamydia trachomatis , although the infectious agent is not always eliminated as judged by immunofluorescence. Inclusion conjunctivitis caused by Chlamydia trachomatis . Uncomplicated urethral, endocervical or rectal infections in adults caused by Chlamydia trachomatis . Nongonococcal urethritis caused by Ureaplasma urealyticum . Relapsing fever due to Borrelia recurrentis . Doxycycline is also indicated for the treatment of infections caused by the following gram-negative microorganisms: Chancroid caused by Haemophilus ducreyi . Plague due to Yersinia pestis . Tularemia due to Francisella tularensis . Cholera caused by Vibrio cholerae . Campylobacter fetus infections caused by Campylobacter fetus . Brucellosis due to Brucella species (in conjunction with streptomycin). Bartonellosis due to Bartonella bacilliformis . Granuloma inguinale caused by Calymmatobacterium granulomatis . Because many strains of the following groups of microorganisms have been shown to be resistant to doxycycline, culture and susceptibility testing are recommended. Doxycycline is indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug: Escherichia coli Enterobacter aerogenes Shigella species Acinetobacter species Respiratory tract infections caused by Haemophilus influenzae . Respiratory tract and urinary tract infections caused by Klebsiella species. Doxycycline is indicated for treatment of infections caused by the following gram-positive microorganisms when bacteriologic testing indicates appropriate susceptibility to the drug: Upper respiratory infections caused by Streptococcus pneumoniae . Anthrax due to Bacillus anthracis , including inhalational anthrax (post-exposure): to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis . When penicillin is contraindicated, doxycycline is an alternative drug in the treatment of the following infections: Uncomplicated gonorrhea caused by Neisseria gonorrhoeae . Syphilis caused by Treponema pallidum . Yaws caused by Treponema pertenue . Listeriosis due to Listeria monocytogenes . Vincent’s infection caused by Fusobacterium fusiforme . Actinomycosis caused by Actinomyces israelii . Infections caused by Clostridium species. In acute intestinal amebiasis, doxycycline may be a useful adjunct to amebicides. In severe acne, doxycycline may be useful adjunctive therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION THE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF DOXYCYCLINE DIFFERS FROM THAT OF THE OTHER TETRACYCLINES. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS. Adults: The usual dose of oral doxycycline is 200 mg on the first day of treatment (administered 100 mg every 12 hours or 50 mg every 6 hours) followed by a maintenance dose of 100 mg/day. The maintenance dose may be administered as a single-dose or as 50 mg every 12 hours. In the management of more severe infections (particularly chronic infections of the urinary tract), 100 mg every 12 hours is recommended. For pediatric patients above eight years of age: The recommended dosage schedule for pediatric patients weighing 100 pounds or less is 2 mg/lb of body weight divided into two doses on the first day of treatment, followed by 1 mg/lb of body weight given as a single daily dose or divided into two doses, on subsequent days. For more severe infections up to 2 mg/lb of body weight may be used. For pediatric patients over 100 pounds the usual adult dose should be used. Uncomplicated gonococcal infections in adults (except anorectal infections in men): 100 mg, by mouth, twice a day for 7 days. As an alternate single visit dose, administer 300 mg stat followed in one hour by a second 300 mg dose. Acute epididymo-orchitis caused by N. gonorrhoeae : 100 mg, by mouth, twice a day for at least 10 days. Primary and secondary syphilis: 300 mg a day in divided doses for at least 10 days. Uncomplicated urethral, endocervical, or rectal infection in adults caused by Chlamydia trachomatis : 100 mg, by mouth, twice a day for at least 7 days. Nongonococcal urethritis caused by C. trachomatis and U. urealyticum : 100 mg, by mouth, twice a day for at least 7 days. Acute epididymo-orchitis caused by C. trachomatis : 100 mg, by mouth, twice a day for at least 10 days. Inhalational anthrax (post-exposure): ADULTS: 100 mg of doxycycline, by mouth, twice a day for 60 days. CHILDREN: weighing less than 100 pounds (45 kg); 1 mg/lb (2.2 mg/kg) of body weight, by mouth, twice a day for 60 days. Children weighing 100 pounds or more should receive the adult dose. When used in streptococcal infections, therapy should be continued for 10 days. Administration of adequate amounts of fluid along with capsule and tablet forms of drugs in the tetracycline class is recommended to wash down the drugs and reduce the risk of esophageal irritation and ulceration (see ADVERSE REACTIONS ). If gastric irritation occurs, doxycycline may be given with food. Ingestion of a high fat meal has been shown to delay the time to peak plasma concentrations by an average of one hour and 20 minutes. However, in the same study, food enhanced the average peak concentration by 7.5% and the area under the curve by 5.7%.
