Drug Catalog - Product Detail
DORZOLAMIDE OPHTHALMIC SOLUTION SOL 20MG/ML 10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0485-10 | BAUSCH HEALTH US | 10 | 2% | SOLUTION |
PACKAGE FILES
Generic Name
DORZOLAMIDE HCL
Substance Name
DORZOLAMIDE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
ANDA090143
Description
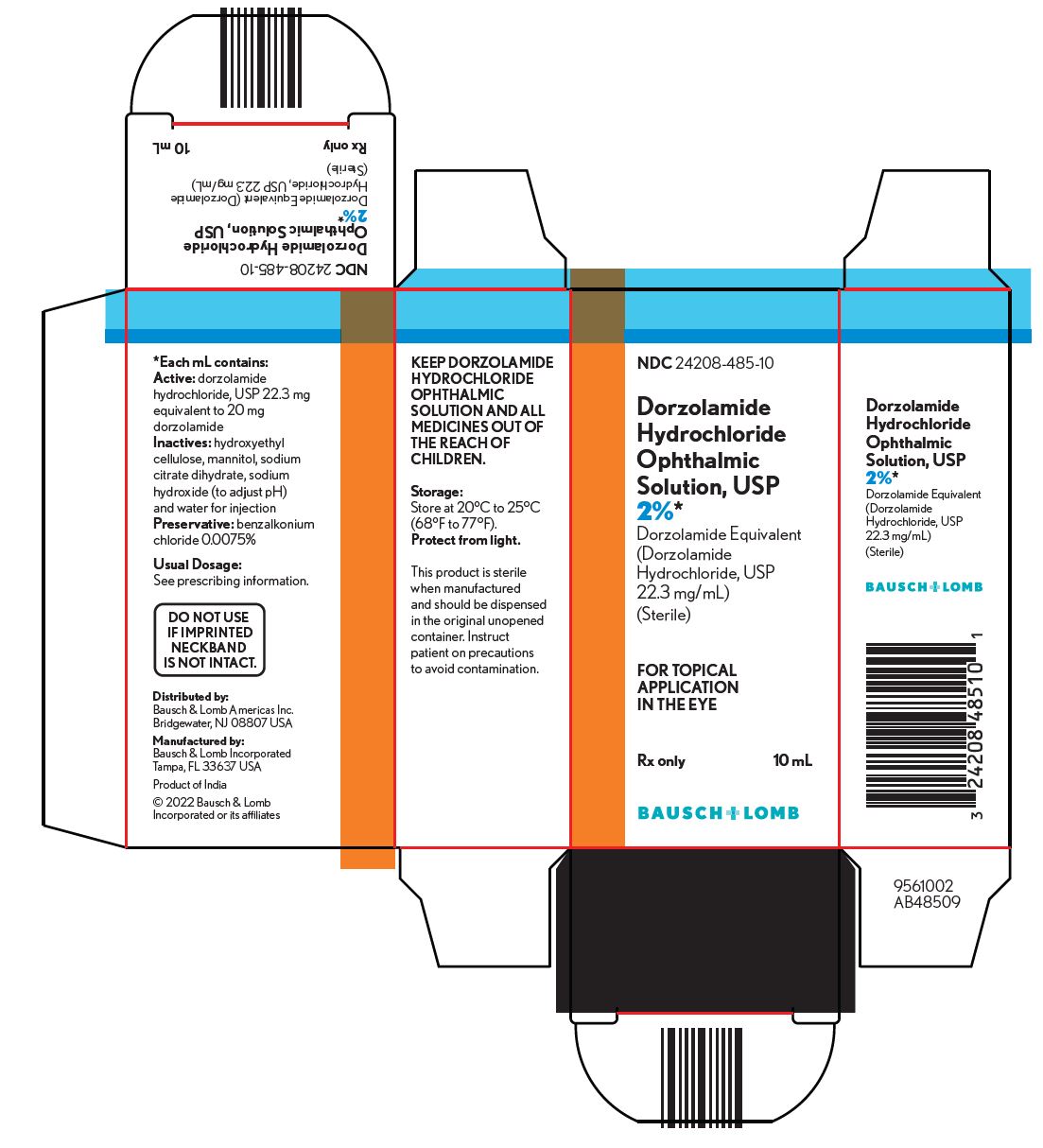
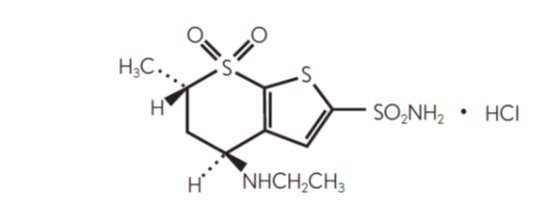
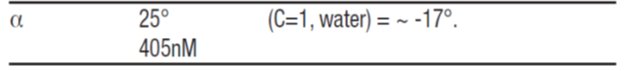
11 DESCRIPTION Dorzolamide hydrochloride ophthalmic solution, USP is a carbonic anhydrase inhibitor formulated for topical ophthalmic use. Dorzolamide hydrochloride USP is described chemically as: (4 S-trans )-4-(ethylamino)-5,6-dihydro-6-methyl-4 H -thieno[2,3- b ]thiopyran-2-sulfonamide 7,7-dioxide monohydrochloride. Dorzolamide hydrochloride is optically active. The specific rotation is: Its empirical formula is C 10 H 16 N 2 O 4 S 3 ∙HCl and its structural formula is: Dorzolamide hydrochloride USP has a molecular weight of 360.9 and a melting point of about 264°C. It is a white to off-white, crystalline powder, which is soluble in water and slightly soluble in methanol and ethanol. Dorzolamide hydrochloride ophthalmic solution, USP is supplied as a sterile, isotonic, buffered, slightly viscous, aqueous solution of dorzolamide hydrochloride USP. The pH of the solution is approximately 5.6, and the osmolarity is 260-330 mOsM. Each mL of dorzolamide hydrochloride ophthalmic solution USP, 2% contains 20 mg dorzolamide (equivalent to 22.3 mg of dorzolamide hydrochloride USP). Inactive ingredients are hydroxyethyl cellulose, mannitol, sodium citrate dihydrate, sodium hydroxide (to adjust pH) and water for injection. Benzalkonium chloride 0.0075% is added as a preservative. Chem1 Chem
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Dorzolamide hydrochloride ophthalmic solution USP, 2% is supplied sterile in a white low density polyethylene (LDPE) bottle with a controlled drop tip and an orange polypropylene cap in the following sizes: NDC 24208-485-05 - 5 mL in 7.5 mL bottle NDC 24208-485-10 - 10 mL in 10 mL bottle Storage Store dorzolamide hydrochloride ophthalmic solution, USP at 20°C to 25°C (68°F to 77°F). Protect from light. After opening, dorzolamide hydrochloride ophthalmic solution, USP can be used until the expiration date on the bottle.
Indications & Usage
1 INDICATIONS AND USAGE Dorzolamide hydrochloride ophthalmic solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. Dorzolamide hydrochloride ophthalmic solution is a carbonic anhydrase inhibitor indicated for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. ( 1 )
Dosage and Administration
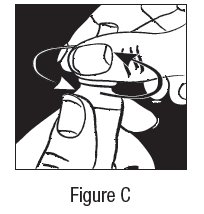
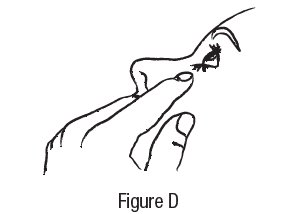
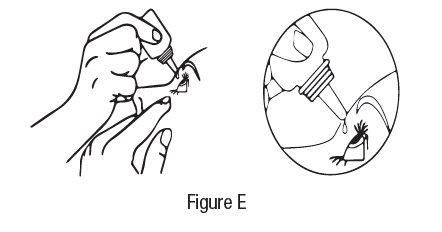
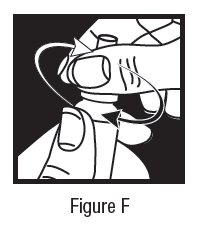
2 DOSAGE AND ADMINISTRATION The dose is one drop of dorzolamide hydrochloride ophthalmic solution in the affected eye(s) three times daily. Dorzolamide hydrochloride ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart. The dose is one drop of dorzolamide hydrochloride ophthalmic solution in the affected eye(s) three times daily. Dorzolamide hydrochloride ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. ( 2 )
