Drug Catalog - Product Detail
DIVALPROEX SODIUM SPRINKLES CP 125MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55111-0532-01 | DR.REDDY'S LABORATORIES, INC. | 100 | 125MG | CAPSULE |
PACKAGE FILES




Generic Name
DIVALPROEX SODIUM
Substance Name
DIVALPROEX SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078979
Description
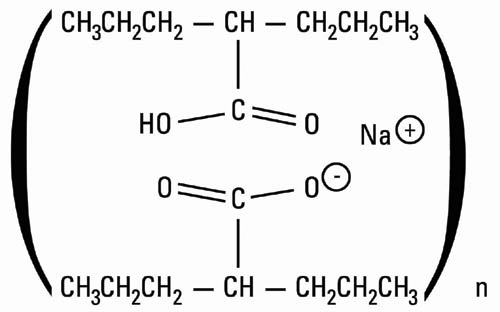
11 DESCRIPTION Divalproex sodium USP is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship and formed during the partial neutralization of valproic acid with 0.5 equivalent of sodium hydroxide. Chemically it is designated as sodium hydrogen bis(2-propylpentanoate). Divalproex sodium USP has the following structure: Divalproex sodium USP occurs as a white to off white powder with a characteristic odor, very soluble in chloroform, freely soluble in methanol and ethyl ether, soluble in acetone, practically insoluble in acetonitrile. Divalproex sodium delayed-release capsules, USP are for oral administration. Divalproex sodium delayed-release capsules, USP contain specially coated particles of divalproex sodium USP equivalent to 125 mg of valproic acid in a hard gelatin capsule. Inactive Ingredients Divalproex sodium delayed-release capsules, USP: black iron oxide, D&C Red 28, ethyl cellulose, FD&C Blue 1, gelatin, hypromellose, magnesium stearate, sugar sphere, titanium dioxide, and triethyl citrate NF. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Divalproex sodium delayed-release capsules USP, 125 mg for oral use are white to off-white, coated particles filled in ‘size 1’ hard gelatin capsules of light blue transparent cap imprinted ‘ ↑THIS END UP↑ ’ and white opaque body imprinted ‘RDY 532’ on the body using black ink and are supplied in bottles of 30’s, 100’s, 500’s and unit dose package of 100 (10 x 10). Bottles of 30 NDC 55111-532-30 Bottles of 100 NDC 55111-532-01 Bottles of 500 NDC 55111-532-05 Unit dose package of 100 (10 x 10) NDC 55111-532-78 Recommended Storage : Store at 20°-25°C (68°-77°F); [See USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Divalproex sodium delayed-release capsule is an anti-epileptic drug indicated for : Monotherapy and adjunctive therapy of complex partial seizures and simple and complex absence seizures; adjunctive therapy in patients with multiple seizure types that include absence seizures (1) 1.1 Epilepsy Divalproex sodium delayed-release capsules are indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures. Divalproex sodium delayed-release capsules are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures. Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present. 1.2 Important Limitations Because of the risk to the fetus of decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations, which may occur very early in pregnancy, valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable [see Warnings and Precautions ( 5.2 , 5.3 , 5.4 ), Use in Specific Populations ( 8.1 ), and Patient Counseling Information ( 17 )]. For prophylaxis of migraine headaches, valproate is contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception [see Contraindications (4) ].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Divalproex sodium delayed-release capsules may be swallowed whole or the contents may be sprinkled on soft food. The drug/food mixture should be swallowed immediately (avoid chewing) ( 2.2 ) Safety of doses above 60 mg/kg/day is not established ( 2.1 , 2.2 ) Complex Partial Seizures: Start at 10 to 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day to achieve optimal clinical response; if response is not satisfactory, check valproate plasma level; see full prescribing information for conversion to monotherapy ( 2.1 ) Absence Seizures: Start at 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day until seizure control or limiting side effects (2.1) 2.1 Epilepsy Divalproex sodium delayed-release capsules are administered orally. As divalproex sodium delayed-release capsules dosage is titrated upward, concentrations of clonazepam, diazepam, ethosuximide, lamotrigine, tolbutamide, phenobarbital, carbamazepine, and/or phenytoin may be affected [see Drug Interactions ( 7.2 ) ]. Complex Partial Seizures For adults and children 10 years of age or older. Monotherapy (Initial Therapy) Divalproex sodium delayed-release capsules has not been systematically studied as initial therapy. Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. The probability of thrombocytopenia increases significantly at total trough valproate plasma concentrations above 110 mcg/mL in females and 135 mcg/mL in males. The benefit of improved seizure control with higher doses should be weighed against the possibility of a greater incidence of adverse reactions. Conversion to Monotherapy Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. Concomitant antiepilepsy drug (AED) dosage can ordinarily be reduced by approximately 25% every 2 weeks. This reduction may be started at initiation of divalproex sodium delayed-release capsules therapy, or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction. The speed and duration of withdrawal of the concomitant AED can be highly variable, and patients should be monitored closely during this period for increased seizure frequency. Adjunctive Therapy Divalproex sodium delayed-release capsules may be added to the patient's regimen at a dosage of 10 to 15 mg/kg/day. The dosage may be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. If the total daily dose exceeds 250 mg, it should be given in divided doses. In a study of adjunctive therapy for complex partial seizures in which patients were receiving either carbamazepine or phenytoin in addition to valproate, no adjustment of carbamazepine or phenytoin dosage was needed [see Clinical Studies (14) ]. However, since valproate may interact with these or other concurrently administered AEDs as well as other drugs, periodic plasma concentration determinations of concomitant AEDs are recommended during the early course of therapy [see Drug Interactions (7)] . Simple and Complex Absence Seizures The recommended initial dose is 15 mg/kg/day, increasing at one week intervals by 5 to 10 mg/kg/day until seizures are controlled or side effects preclude further increases. The maximum recommended dosage is 60 mg/kg/day. If the total daily dose exceeds 250 mg, it should be given in divided doses. A good correlation has not been established between daily dose, serum concentrations, and therapeutic effect. However, therapeutic valproate serum concentrations for most patients with absence seizures are considered to range from 50 to 100 mcg/mL. Some patients may be controlled with lower or higher serum concentrations [see Clinical Pharmacology ( 12.3 ) ]. As the divalproex sodium delayed-release capsules dosage is titrated upward, blood concentrations of phenobarbital and/or phenytoin may be affected [see Drug Interactions ( 7.2 ) ]. Antiepilepsy drugs should not be abruptly discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In epileptic patients previously receiving valproic acid therapy, divalproex sodium delayed-release capsules should be initiated at the same daily dose and dosing schedule. After the patient is stabilized on divalproex sodium delayed-release capsules, a dosing schedule of two or three times a day may be elected in selected patients. 2.2 General Dosing Advice Dosing in Elderly Patients Due to a decrease in unbound clearance of valproate and possibly a greater sensitivity to somnolence in the elderly, the starting dose should be reduced in these patients. Dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence. The ultimate therapeutic dose should be achieved on the basis of both tolerability and clinical response [see Warnings and Precautions (5.14) , Use in Specific Populations (8.5) and Clinical Pharmacology (12.3) ]. Dose-Related Adverse Reactions The frequency of adverse effects (particularly elevated liver enzymes and thrombocytopenia) may be dose-related. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of ≥ 110 mcg/mL (females) or ≥ 135 mcg/mL (males) [see Warnings and Precautions (5.8 )]. The benefit of improved therapeutic effect with higher doses should be weighed against the possibility of a greater incidence of adverse reactions. G.I. Irritation Patients who experience G.I. irritation may benefit from administration of the drug with food or by slowly building up the dose from an initial low level. Administration of Divalproex Sodium Delayed-release Capsules Divalproex sodium delayed-release capsules may be swallowed whole or may be administered by carefully opening the capsule and sprinkling the entire contents on a small amount (teaspoonful) of soft food such as applesauce or pudding. The drug/food mixture should be swallowed immediately (avoid chewing) and not stored for future use. Each capsule is oversized to allow ease of opening. 2.3 Dosing in Patients Taking Rufinamide Patients stabilized on rufinamide before being prescribed valproate should begin valproate therapy at a low dose, and titrate to a clinically effective dose [see Drug Interactions (7.2) ].
