Drug Catalog - Product Detail
DIPYRIDAMOLE TB 25MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 64980-0133-01 | RISING PHARMACEUTICALS | 100 | 25MG | TABLET |
PACKAGE FILES




Generic Name
DIPYRIDAMOLE
Substance Name
DIPYRIDAMOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040733
Description
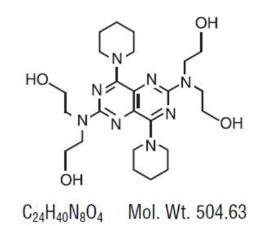
DESCRIPTION Dipyridamole USP is a platelet inhibitor chemically described as 2,2',2",2"'-[(4,8- Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula: Dipyridamole is an odorless yellow crystalline powder, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and practically insoluble in water. Dipyridamole Tablets, USP for oral administration contain: Active Ingredient TABLETS 25 mg, 50 mg, and 75 mg: dipyridamole USP 25 mg, 50 mg and 75 mg, respectively. Inactive Ingredients TABLETS 25 mg, 50 mg, and 75 mg: corn starch, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, and titanium dioxide. structural formula
How Supplied
HOW SUPPLIED Dipyridamole Tablets, USP are available as round, white, film-coated tablets of 25 mg, 50 mg, and 75 mg coded 81/SL, 82/SL, and 83/SL, respectively. They are available in bottles of 100 tablets as indicated below: 25 mg Tablets (NDC 64980-133-01) 50 mg Tablets (NDC 64980-134-01) 75 mg Tablets (NDC 64980-135-01) They are available in bottles of 1000 tablets as indicated below: 25 mg Tablets (NDC 64980-133-10) 50 mg Tablets (NDC 64980-134-10) 75 mg Tablets (NDC 64980-135-10) Store at 25°C (77°F); excursions permitted to 15°-30 ° C (59°-86°F) [see USP Controlled Room Temperature]. Keep out of reach of children. For medical inquiries, please visit the website www.risingpharma.com or call 1-866-562-4597. Distributed by: Rising Pharmaceuticals, Inc., Saddle Brook, NJ 07663 USA Manufactured by: Murty Pharmaceuticals, Inc., Lexington, KY 40509 USA Revised: December 2019 P112-03
Indications & Usage
INDICATIONS AND USAGE Dipyridamole tablets are indicated as an adjunct to coumarin anticoagulants in the prevention of postoperative thromboembolic complications of cardiac valve replacement.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adjunctive Use in Prophylaxis of Thromboembolism after Cardiac Valve Replacement. The recommended dose is 75 mg to 100 mg four times daily as an adjunct to the usual warfarin therapy. Please note that aspirin is not to be administered concomitantly with coumarin anticoagulants.
