Drug Catalog - Product Detail
DILTIAZEM HCL ER (CD) CP 360MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00228-2918-09 | ACTAVIS PHARMA | 90 | 360MG | CAPSULE |
PACKAGE FILES

Generic Name
DILTIAZEM HYDROCHLORIDE
Substance Name
DILTIAZEM HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202463
Description
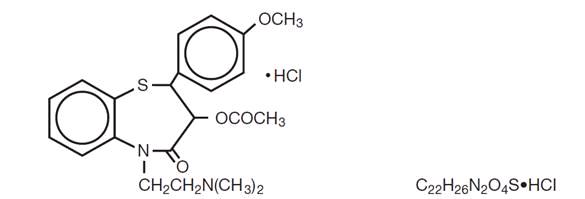
DESCRIPTION Diltiazem hydrochloride extended-release capsules, USP are a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride, USP is 1,5-Benzothiazepin-4(5 H )-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride,(+)- cis -. The chemical structure is: Diltiazem hydrochloride, USP is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol, and chloroform. It has a molecular weight of 450.98. Diltiazem hydrochloride, USP is formulated as a once-a-day extended release capsule containing 360 mg diltiazem hydrochloride, USP (equivalent to 330.9 mg diltiazem). Each diltiazem hydrochloride extended-release capsule, USP, for oral administration, contains the following inactive ingredients: ammonio methacrylate copolymer, type A, ammonio methacrylate copolymer, type B, hydroxypropyl cellulose, sodium lauryl sulfate, sugar spheres, talc, triethyl citrate. The capsule shells contain FD&C Blue No.1, gelatin and titanium dioxide. The capsules are imprinted with black ink which contain black iron oxide, potassium hydroxide, propylene glycol, and shellac. This product meets Dissolution Test 20. e1bfe0c2-figure-01
How Supplied
HOW SUPPLIED Diltiazem hydrochloride extended-release capsules, USP are supplied as follows: 360 mg — Each #00 capsule with blue opaque cap and white opaque body imprinted with and 2918 on both cap and body in black ink contains 360 mg of diltiazem hydrochloride, USP. Capsules are supplied in bottles of 90 (NDC 0228-2918-09). Dispense in tight, light-resistant containers as defined in the USP. Storage Conditions: Store at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [see USP Controlled Room Temperature]. Avoid excessive humidity. Manufactured By: Teva Pharmaceuticals USA, Inc. Parsippany, NJ 07054 Rev. B 5/2025 e1bfe0c2-figure-02
Indications & Usage
INDICATIONS AND USAGE Diltiazem hydrochloride extended-release capsules are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive medications. Diltiazem hydrochloride extended-release capsules are indicated for the management of chronic stable angina and angina due to coronary artery spasm.
Dosage and Administration
DOSAGE AND ADMINISTRATION Patients controlled on diltiazem alone or in combination with other medications may be switched to diltiazem hydrochloride extended-release capsules at the nearest equivalent total daily dose. Higher doses of diltiazem hydrochloride extended-release may be needed in some patients. Monitor patients closely. Subsequent titration to higher or lower doses may be necessary. There is limited general clinical experience with doses above 360 mg, but doses to 540 mg have been studied in clinical trials. The incidence of side effects increases as the dose increases with first-degree AV block, dizziness, and sinus bradycardia bearing the strongest relationship to dose. Hypertension: Adjust dosage to individual patient needs. When used as monotherapy, reasonable starting doses are 180 mg to 240 mg once daily, although some patients may respond to lower doses. Maximum antihypertensive effect is usually observed by 14 days of chronic therapy; therefore, schedule dosage adjustments accordingly. The usual dosage range studied in clinical trials was 240 mg to 360 mg once daily. Individual patients may respond to higher doses of up to 480 mg once daily. Angina: Dosages for the treatment of angina should be adjusted to each patient’s needs, starting with a dose of 120 mg or 180 mg once daily. Individual patients may respond to higher doses of up to 480 mg once daily. When necessary, titration may be carried out over a 7- to 14-day period. Concomitant Use with Other Cardiovascular Agents: Sublingual NTG: May be taken as required to abort acute anginal attacks during diltiazem hydrochloride therapy. Prophylactic Nitrate Therapy: Diltiazem hydrochloride may be safely coadministered with short- and long-acting nitrates. Beta-Blockers: (see WARNINGS and PRECAUTIONS ). Antihypertensives: Diltiazem hydrochloride has an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride or the concomitant antihypertensives may need to be adjusted when adding one to the other.
