Drug Catalog - Product Detail
DIHYDROERGOTAMINE MESYLATE 4MG/ML NAS SPR 1ML X 8
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68682-0357-10 | OCEANSIDE PHARMACEUTICALS | 1 | 4MG/ML | SOLUTION |
PACKAGE FILES
Generic Name
DIHYDROERGOTAMINE MESYLATE
Substance Name
DIHYDROERGOTAMINE MESYLATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
NASAL
Application Number
NDA020148
Description
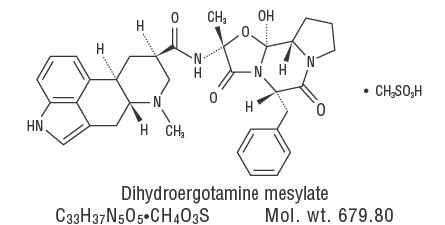
DESCRIPTION Dihydroergotamine mesylate is ergotamine hydrogenated in the 9,10 position as the mesylate salt. Dihydroergotamine Mesylate Nasal Spray is known chemically as ergotaman-3’, 6’, 18-trione, 9,10-dihydro-12’-hydroxy-2’-methyl-5’- (phenylmethyl)-, (5’α)-, monomethane-sulfonate. Its molecular weight is 679.78 and its empirical formula is C 33 H 37 N 5 O 5 •CH 4 O 3 S. The chemical structure is: Dihydroergotamine Mesylate Nasal Spray is provided for intranasal administration as a clear, colorless to light yellow aqueous solution in an amber glass vial containing: dihydroergotamine mesylate 4 mg caffeine, anhydrous 10 mg dextrose, anhydrous 50 mg carbon dioxide qs purified water qs 1 mL Each milliliter contains Dihydroergotamine mesylate 4 mg (equivalent to 3.43 mg dihydroergotamine) Chemical Structure
How Supplied
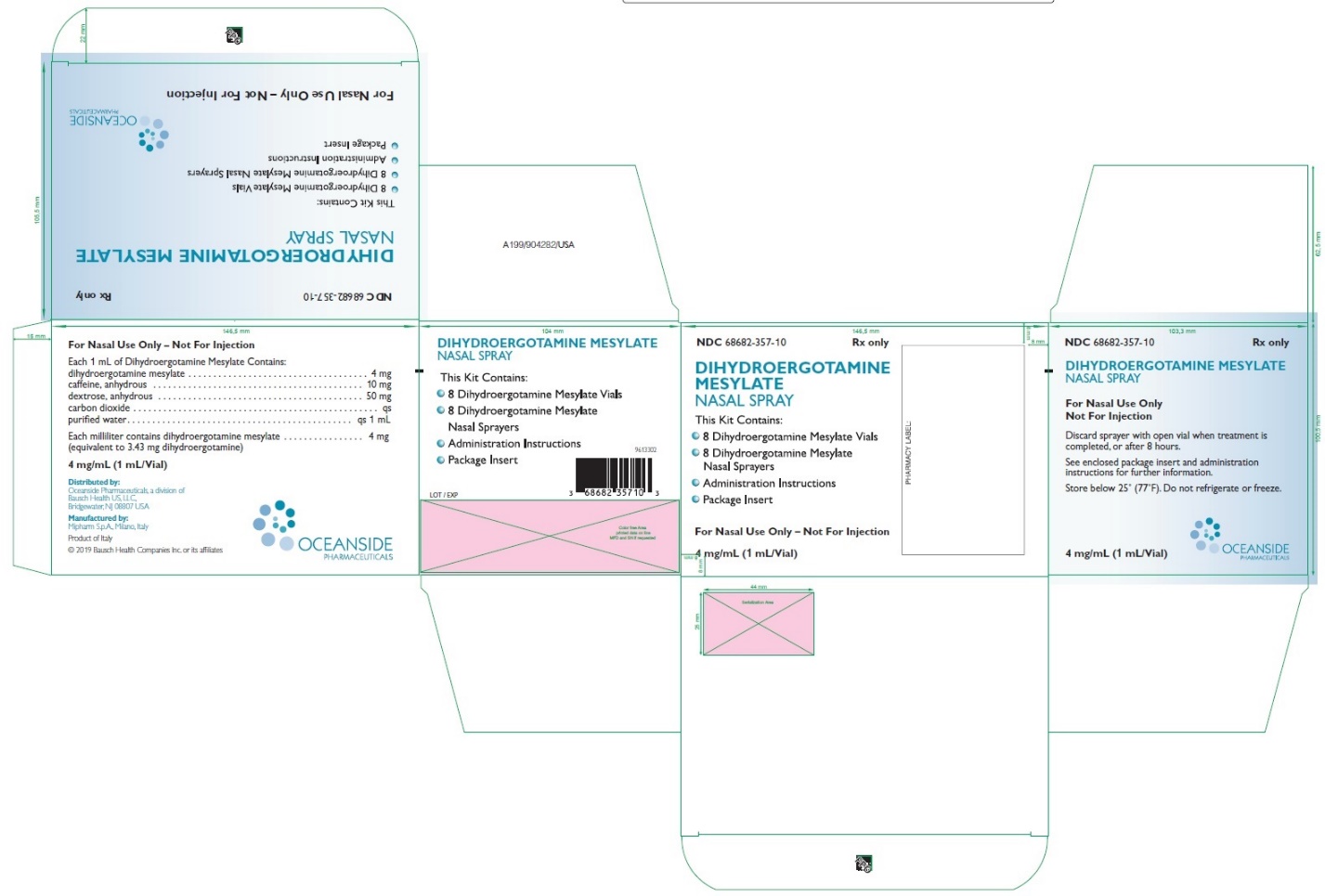
HOW SUPPLIED Dihydroergotamine Mesylate Nasal Spray is available (as a clear, colorless to light yellow aqueous solution) in 3.5 mL amber glass vials containing 4 mg of dihydroergotamine mesylate. Dihydroergotamine Mesylate Nasal Spray is provided as a package of 8 units, administration instruction sheet, and one package insert. Each unit consists of one vial and one sprayer (NDC 68682-357-10). Store below 25°C (77°F). Do not refrigerate or freeze. * Trademark of PDR Network, LLC Distributed by: Oceanside Pharmaceuticals, a division of Bausch Health US, LLC Bridgewater, NJ 08807 USA Manufactured by: Mipharm, S.p.A. Milano, Italy © 2022 Bausch Health Companies Inc. or its affiliates Rev. 09/2022 9606502
Indications & Usage
INDICATIONS AND USAGE Dihydroergotamine Mesylate Nasal Spray is indicated for the acute treatment of migraine headaches with or without aura. Dihydroergotamine Mesylate Nasal Spray is not intended for the prophylactic therapy of migraine or for the management of hemiplegic or basilar migraine.
Dosage and Administration
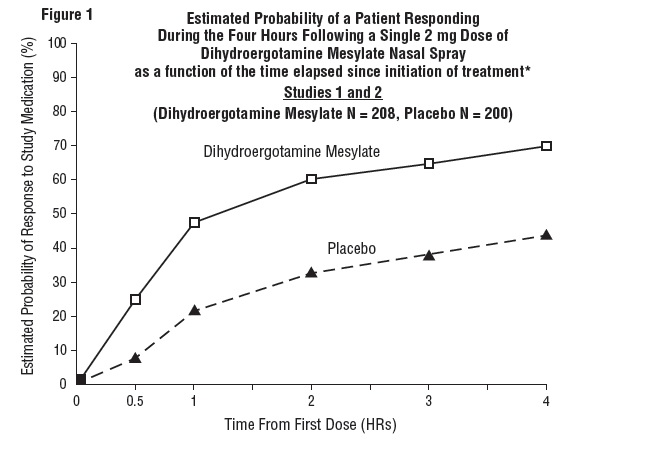
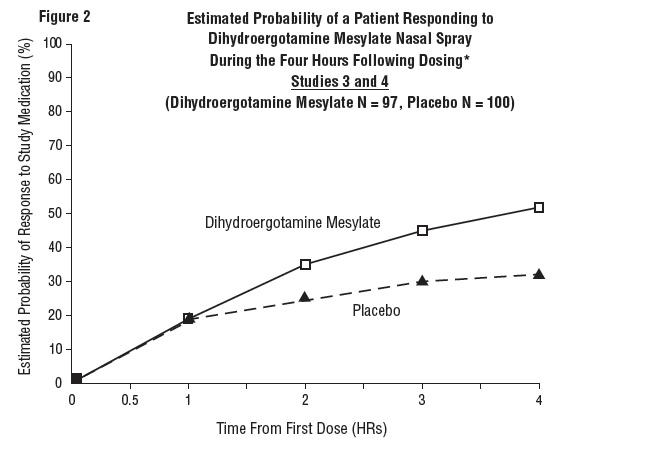
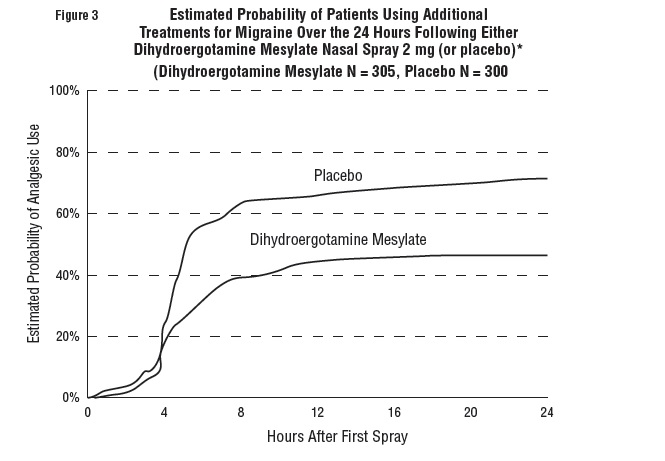
DOSAGE AND ADMINISTRATION The solution used in Dihydroergotamine Mesylate Nasal Spray (4 mg/mL) is intended for intranasal use and must not be injected. In clinical trials, Dihydroergotamine Mesylate Nasal Spray has been effective for the acute treatment of migraine headaches with or without aura. One spray (0.5 mg) of Dihydroergotamine Mesylate Nasal Spray should be administered in each nostril. Fifteen minutes later, an additional one spray (0.5 mg) of Dihydroergotamine Mesylate Nasal Spray should be administered in each nostril, for a total dosage of four sprays (2 mg) of Dihydroergotamine Mesylate Nasal Spray. Studies have shown no additional benefit from acute doses greater than 2 mg for a single migraine administration. The safety of doses greater than 3 mg in a 24-hour period and 4 mg in a 7‑day period has not been established. Dihydroergotamine Mesylate Nasal Spray, should not be used for chronic daily administration. Prior to administration, the pump must be primed (i.e., squeeze 4 times) before use (see administration instructions). Once the nasal spray applicator has been prepared, it should be discarded (with any remaining drug in opened vial) after 8 hours. Prior to administration, the pump must be primed (i.e., squeeze 4 times) before use (see administration instructions). Once the nasal spray applicator has been prepared, it should be discarded (with any remaining drug in opened vial after 8 hours).
