Drug Catalog - Product Detail
DIGOXIN TB 0.25MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 57664-0441-18 | SUN PHARMACEUTICALS | 1000 | 250MCG | TABLET |
PACKAGE FILES
Generic Name
DIGOXIN
Substance Name
DIGOXIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA076363
Description
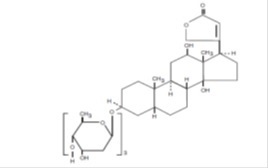
11 DESCRIPTION Digoxin is one of the cardiac (or digitalis) glycosides, a closely related group of drugs having in common specific effects on the myocardium. These drugs are found in a number of plants. Digoxin is extracted from the leaves of Digitalis lanata . The term “digitalis” is used to designate the whole group of glycosides. The glycosides are composed of 2 portions: a sugar and a cardenolide (hence “glycosides”). Digoxin is described chemically as (3β,5β,12β)-3-[( O -2,6-dideoxy-β- D - ribo -hexopyranosyl(1→4)- O -2,6- dideoxy-β- D - ribo -hexopyranosyl-(1→4)-2,6-dideoxy-β- D - ribo- hexopyranosyl)oxy]-12,14-dihydroxy-card-20(22)-enolide. Its molecular formula is C 41 H 64 O 14 , its molecular weight is 780.95, and its structural formula is: Digoxin exists as odorless white crystals that melt with decomposition above 230°C. The drug is practically insoluble in water and in ether; slightly soluble in diluted (50%) alcohol and in chloroform; and freely soluble in pyridine. Digoxin is supplied as 125 mcg (0.125 mg) or 250 mcg (0.25 mg) tablets for oral administration. Each tablet contains the labeled amount of digoxin USP and the following inactive ingredients: lactose monohydrate, hydroxypropyl methylcellulose, magnesium stearate. In addition, the dye used in the 0.125 mg tablets is D & C Yellow No. 10 aluminum lake. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Digoxin Tablets USP, 125 micrograms (0.125 mg): Bottles of 100 with child-resistant cap (NDC 57664-437-88) and 1000 (NDC 57664-437-18). Round, biconvex, yellow tablets, debossed with "437" on one side and scored on other side. Digoxin Tablets USP, 250 micrograms (0.25 mg): Bottles of 100 with child-resistant cap (NDC 57664-441-88) and 1000 (NDC 57664-441-18). Round, biconvex, white tablets, debossed with "441" on one side and scored on other side. Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature] in a dry place and protect from light. Keep out of reach of children. Dispense in tight, light-resistant container.
Indications & Usage
1 INDICATIONS AND USAGE Digoxin is a cardiac glycoside indicated for: Treatment of mild to moderate heart failure in adults (1.1) . Increasing myocardial contractility in pediatric patients with heart failure (1.2) . Control of resting ventricular rate in patients with chronic atrial fibrillation in adults (1.3) . 1.1 Heart Failure in Adults Digoxin is indicated for the treatment of mild to moderate heart failure in adults. Digoxin increases left ventricular ejection fraction and improves heart failure symptoms as evidenced by improved exercise capacity and decreased heart failure-related hospitalizations and emergency care, while having no effect on mortality. Where possible, digoxin should be used in combination with a diuretic and an angiotensin-converting enzyme (ACE) inhibitor. 1.2 Heart Failure in Pediatric Patients Digoxin increases myocardial contractility in pediatric patients with heart failure. 1.3 Atrial Fibrillation in Adults Digoxin is indicated for the control of ventricular response rate in adult patients with chronic atrial fibrillation.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Digoxin dose is based on patient-specific factors (age, lean body weight, renal function, etc.). See full prescribing information. Monitor for toxicity and therapeutic effect (2) . 2.1 Important Dosing and Administration Information In selecting a digoxin dosing regimen, it is important to consider factors that affect digoxin blood levels (e.g., body weight, age, renal function, concomitant drugs) since toxic levels of digoxin are only slightly higher than therapeutic levels. Dosing can be either initiated with a loading dose followed by maintenance dosing if rapid titration is desired or initiated with maintenance dosing without a loading dose. Consider interruption or reduction in digoxin dose prior to electrical cardioversion [see Warnings and Precautions (5.4)]. Use digoxin solution to obtain the appropriate dose in infants, young pediatric patients, or patients with very low body weight. 2.2 Loading Dosing Regimen in Adults and Pediatric Patients For adults and pediatric patients if a loading dosage is to be given, administer half the total loading dose initially, then ¼ the loading dose every 6 to 8 hours twice, with careful assessment of clinical response and toxicity before each dose. The recommended loading dose is displayed in Table 1. Table 1. Recommended Digoxin Oral Loading Dose Age Total Oral Loading Dose (mcg/kg) Administer half the total loading dose initially, then 1/4 the loading dose every 6 to 8 hours twice 5 to 10 years 20-45 Adult and pediatric patients over 10 years 10-15 mcg= microgram 2.3 Maintenance Dosing in Adults and Pediatric Patients Over 10 Years Old The maintenance dose is based on lean body weight, renal function, age, and concomitant products [see Clinical Pharmacology (12.3)]. The recommended starting maintenance dose in adults and pediatric patients over 10 years old with normal renal function is given in Table 2. Doses may be increased every 2 weeks according to clinical response, serum drug levels, and toxicity. Table 2. Recommended Starting Digoxin Maintenance Dosage in Adults and Pediatric Patients Over 10 Years Old Age Total Oral Maintenance Dose, mcg/kg/day (given once daily) Adults and pediatric patients over 10 years 3.4 to 5.1 mcg=microgram Table 3 provides the recommended (once daily) maintenance dose for adults and pediatric patients over 10 years old (to be given once daily) according to lean body weight and renal function. The doses are based on studies in adult patients with heart failure. Alternatively, the maintenance dose may be estimated by the following formula (peak body stores lost each day through elimination): Total Maintenance Dose = Loading Dose (i.e., Peak Body Stores) x % Daily Loss/100 (% Daily Loss = 14 + Creatinine clearance/5) Reduce the dose of digoxin in patients whose lean weight is an abnormally small fraction of their total body mass because of obesity or edema. Table 3. Recommended Maintenance Dose (in micrograms given once daily) of Digoxin in Pediatric Patients Over 10 Years Old and Adults by Lean Body Weight and by Renal Functionª Corrected Creatinine Clearance b Lean Body Weight d Number of days Before Steady State Achieved c Kg 40 50 60 70 80 90 100 10 mL/min 62.5* 125 125 187.5 187.5 187.5 250 19 20 mL/min 125 125 125 187.5 187.5 250 250 16 30 mL/min 125 125 187.5 187.5 250 250 312.5 14 40 mL/min 125 187.5 187.5 250 250 312.5 312.5 13 50 mL/min 125 187.5 187.5 250 250 312.5 312.5 12 60 mL/min 125 187.5 250 250 312.5 312.5 375 11 70 mL/min 187.5 187.5 250 250 312.5 375 375 10 80 mL/min 187.5 187.5 250 312.5 312.5 375 437.5 9 90 mL/min 187.5 250 250 312.5 375 437.5 437.5 8 100 mL/min 187.5 250 312.5 312.5 375 437.5 500 7 a Doses are rounded to the nearest dose possible using whole digoxin tablets. Recommended doses approximately 30 percent lower than the calculated dose are designated with an *. Monitor digoxin levels in patients receiving these initial doses and increase dose if needed. b For adults, creatinine clearance was corrected to 70-kg body weight or 1.73 m 2 body surface area. If only serum creatinine concentrations (Scr) are available, a corrected Ccr may be estimated in men as (140 – Age)/Scr. For women, this result should be multiplied by 0.85. For pediatric patients, the modified Schwartz equation may be used. The formula is based on height in cm and Scr in mg/dL where k is a constant. Ccr is corrected to 1.73 m 2 body surface area. During the first year of life, the value of k is 0.33 for pre-term babies and 0.45 for term infants. The k is 0.55 for pediatric patients and adolescent girls and 0.7 for adolescent boys. GFR (mL/min/1.73 m2) = (k x Height)/Scr c If no loading dose administered. d The doses listed assume average body composition. 2.4 Maintenance Dosing in Pediatric Patients Less Than 10 Years Old The starting maintenance dose for heart failure in pediatric patients less than 10 years old is based on lean body weight, renal function, age, and concomitant products [ see Clinical Pharmacology (12.3)]. The recommended starting maintenance dose for pediatric patients between 5 years and 10 years old is given in Table 4. These recommendations assume the presence of normal renal function. Table 4. Recommended Starting Digoxin Oral Maintenance Dosage in Pediatric Patients Between 5 years and 10 Years Old Age Oral Maintenance Dose, mcg/kg/day 5 years to 10 years 3.2 to 6.4 Twice daily Table 5 provides average daily maintenance dose requirements for pediatric patients between 5 and 10 years old (to be given twice daily) with heart failure based on age, lean body weight, and renal function. Table 5. Recommended Maintenance Dose (in micrograms given TWICE daily) of Digoxin in Pediatric Patients between 5 and 10 Years of Age a Based upon Lean Body Weight and Renal Function a,b Corrected Creatinine Clearance c Lean Body Weight Number of Days Before Steady State Achieved d Kg 20 30 40 50 60 10 mL/min - 62.5 62.5* 125 125 19 20 mL/min 62.5 62.5 125 125 125 16 30 mL/min 62.5 62.5* 125 125 187.5 14 40 mL/min 62.5 62.5* 125 187.5 187.5 13 50 mL/min 62.5 125 125 187.5 187.5 12 60 mL/min 62.5 125 125 187.5 250 11 70 mL/min 62.5 125 187.5 187.5 250 10 80 mL/min 62.5* 125 187.5 187.5 250 9 90 mL/min 62.5* 125 187.5 250 250 8 100 mL/min 62.5* 125 187.5 250 312.5 7 a Recommended are doses to be given twice daily. b The doses are rounded to the nearest dose possible using whole and/or half digoxin tablets. Recommended doses approximately 30 percent lower than the calculated dose are designated with an *. Monitor digoxin levels in patients receiving these initial doses and increase dose if needed. c The modified Schwartz equation may be used to estimate creatinine clearance. See footnote b under Table 3. d If no loading dose administered. 2.5 Monitoring to Assess Safety, Efficacy, and Therapeutic Blood Levels Monitor for signs and symptoms of digoxin toxicity and clinical response. Adjust dose based on toxicity, efficacy, and blood levels. Serum digoxin levels less than 0.5 ng/mL have been associated with diminished efficacy, while levels above 2 ng/mL have been associated with increased toxicity without increased benefit. Interpret the serum digoxin concentration in the overall clinical context, and do not use an isolated measurement of serum digoxin concentration as the basis for increasing or decreasing the digoxin dose. Serum digoxin concentrations may be falsely elevated by endogenous digoxin-like substances [ see Drug Interactions (7.4)]. If the assay is sensitive to these substances, consider obtaining a baseline digoxin level before starting digoxin and correct post-treatment values by the reported baseline level. Obtain serum digoxin concentrations just before the next scheduled digoxin dose or at least 6 hours after the last dose. The digoxin concentration is likely to be 10% to 25% lower when sampled right before the next dose (24 hours after dosing) compared to sampling 8 hours after dosing (using once-daily dosing). However, there will be only minor differences in digoxin concentrations using twice daily dosing whether sampling is done at 8 or 12 hours after a dose. 2.6 Switching from Intravenous Digoxin to Oral Digoxin When switching from intravenous to oral digoxin formulations, make allowances for differences in bioavailability when calculating maintenance dosages (see Table 6). Table 6. Comparison of the Systemic Availability and Equivalent Doses of Oral and Intravenous Digoxin Absolute Bioavailability Equivalent Doses (mcg) Digoxin Tablets 60-80% 62.5 125 250 500 Digoxin Intravenous Injection 100% 50 100 200 400
