Drug Catalog - Product Detail
DICYCLOMINE HCL TB 20MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00527-1282-01 | LANNETT | 100 | 20MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
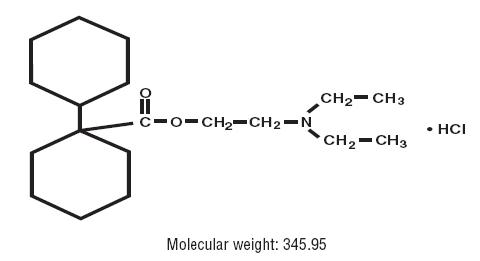
11 DESCRIPTION Dicyclomine hydrochloride is an antispasmodic and anticholinergic (antimuscarinic) agent available in the following dosage forms: Dicyclomine Hydrochloride Capsules, USP for oral use contain 10 mg of dicyclomine hydrochloride, USP. In addition, each capsule contains the following inactive ingredients: lactose monohydrate, calcium sulfate, magnesium stearate, gelatin, FD&C Blue No. 1, and FD&C Red No. 3. Dicyclomine Hydrochloride Tablets, USP for oral use contain 20 mg dicyclomine hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: acacia, pregelatinized starch, anhydrous lactose, compressible sugar, dicalcium phosphate, colloidal silicon dioxide, magnesium stearate, stearic acid, and FD & C Blue No.1 Aluminum Lake. Dicyclomine hydrochloride is [bicyclohexyl]-1-carboxylic acid, 2- (diethylamino) ethyl ester, hydrochloride, with a molecular formula of C 19 H 35 NO 2 •HCl and the following structural formula: Dicyclomine hydrochloride occurs as a fine, white, crystalline, practically odorless powder with a bitter taste. It is soluble in water, freely soluble in alcohol and chloroform, and very slightly soluble in ether. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Dicyclomine Hydrochloride Capsules USP, 10 mg 10 mg blue capsules with a white powder fill, imprinted logo LANNETT on the cap and 0586 on the body, supplied in bottles of 100, 500, and 1000 capsules. Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a well-closed container as defined in the USP. Bottles of 100 capsules: NDC 0527-0586-01 Bottles of 500 capsules: NDC 0527-0586-05 Bottles of 1000 capsules: NDC 0527-0586-10 Dicyclomine Hydrochloride Tablets USP, 20 mg 20 mg blue, round, flat-faced, beveled edge tablets, debossed LAN over 1282, supplied in bottles of 100, 500 and 1000 tablets. To prevent fading, avoid exposure to direct sunlight. Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a well-closed container as defined in the USP. Bottles of 100 tablets: NDC 0527-1282-01 Bottles of 500 tablets: NDC 0527-1282-05 Bottles of 1000 tablets: NDC 0527-1282-10
Indications & Usage
1 INDICATIONS AND USAGE Dicyclomine hydrochloride is indicated for the treatment of patients with functional bowel/irritable bowel syndrome. Dicyclomine hydrochloride is an antispasmodic and anticholinergic (antimuscarinic) agent indicated for the treatment of functional bowel/irritable bowel syndrome ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Dosage must be adjusted to individual patient needs. Dosage for dicyclomine hydrochloride must be adjusted to individual patient needs ( 2 ). If a dose is missed, patients should continue the normal dosing schedule ( 2 ). Oral in adults ( 2.1 ): Starting dose: 20 mg four times a day. After a week treatment with the starting dose, the dose may be escalated to 40 mg four times a day, unless side effects limit dosage escalation Discontinue dicyclomine hydrochloride if efficacy not achieved or side effects require doses less than 80 mg per day after two weeks of treatment 2.1 Oral Dosage and Administration in Adults The recommended initial dose is 20 mg four times a day. After one week treatment with the initial dose, the dose may be increased to 40 mg four times a day unless side effects limit dosage escalation. If efficacy is not achieved within 2 weeks or side effects require doses below 80 mg per day, the drug should be discontinued. Documented safety data are not available for doses above 80 mg daily for periods longer than 2 weeks.
