Drug Catalog - Product Detail
DICLOFENAC SODIUM TOPICAL SOLUTION 1.5% 150ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 52565-0002-05 | TELIGENT | 150 | 1.5% | SOLUTION |
PACKAGE FILES
Generic Name
Substance Name
Product Type
Route
Application Number
Description
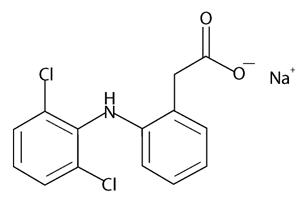
11 DESCRIPTION Diclofenac Sodium Topical Solution USP, 1.5% is a nonsteroidal anti-inflammatory drug, available as a clear, colorless to faintly pink-orange solution for topical application. Diclofenac Sodium Topical Solution USP, 1.5% contains 1.5% w/w diclofenac sodium, a benzene acetic acid derivative that is a nonsteroidal anti-inflammatory drug (NSAID), designated chemically as 2-[(2,6-dichlorophenyl)amino]-benzene acetic acid, monosodium salt. It is a white or slightly yellowish crystalline powder, slightly hygroscopic, and odorless that is freely soluble in methanol, soluble in alcohol, slightly soluble in acetone and sparingly soluble in water. The molecular weight is 318.14. Its molecular formula is C 14 H 10 Cl 2 NNaO 2 and it has the following structural formula: Each 1 mL of solution contains 16.05 mg of diclofenac sodium. The inactive ingredients in Diclofenac Sodium Topical Solution USP, 1.5% include: dimethyl sulfoxide, alcohol (11.79%), propylene glycol, glycerin and purified water.
How Supplied
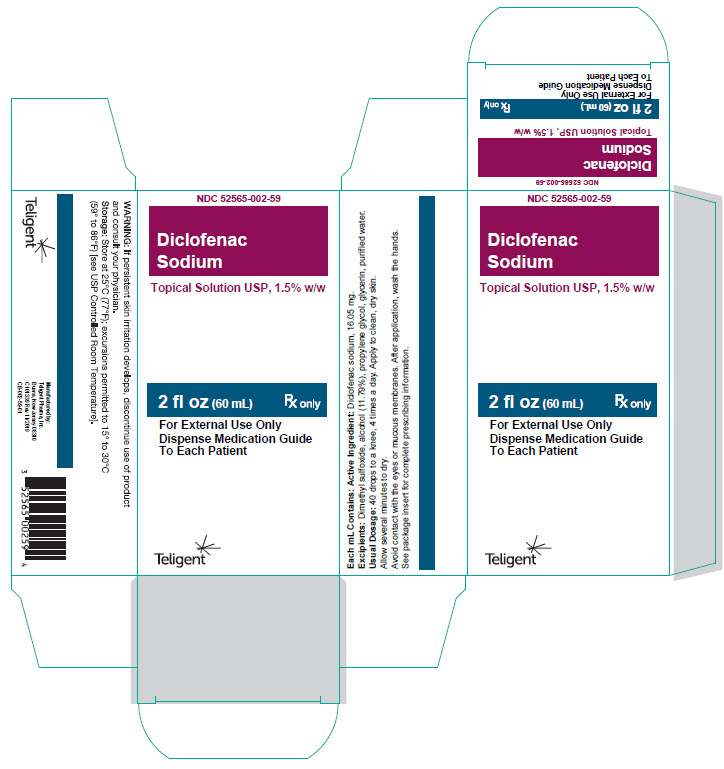
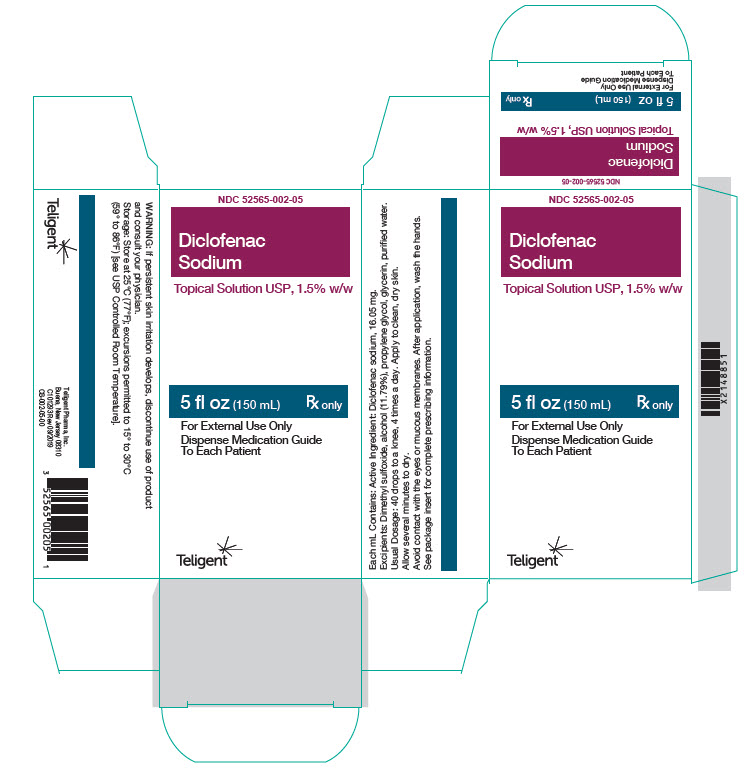
16 HOW SUPPLIED/STORAGE AND HANDLING Diclofenac Sodium Topical Solution USP,1.5% w/w. is supplied as a clear, colorless to faintly pink-orange solution containing 16.05 mg of diclofenac sodium per mL of solution, in a white high-density polyethylene bottle with a white low-density dropper cap. NDC Number and Size 60 mL bottle NDC 52565-002-59 150 mL bottle NDC 52565-002-05 Storage Store at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted between15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Diclofenac Sodium Topical Solution is indicated for the treatment of signs and symptoms of osteoarthritis of the knee(s) ( 1 ). Diclofenac Sodium Topical Solution is a nonsteroidal anti-inflammatory drug indicated for the treatment of signs and symptoms of osteoarthritis of the knee(s). ( 1 )
Dosage and Administration
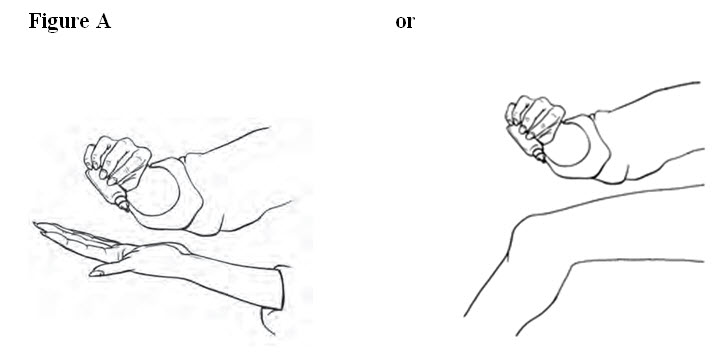
2 DOSAGE AND ADMINISTRATION Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals. The recommended dose is 40 drops on each painful knee, 4 times a day. ( 2 ) Apply Diclofenac Sodium Topical Solution to clean, dry skin. ( 2.1 ) Dispense Diclofenac Sodium Topical Solution 10 drops at a time either directly onto the knee or first into the hand and then onto the knee. Spread Diclofenac Sodium Topical Solution evenly around front, back and sides of the knee. Repeat this procedure until 40 drops have been applied and the knee is completely covered with solution. ( 2.1 ) Wash hands completely after administering the product. Wait until the area is completely dry before covering with clothing or applying sunscreen, insect repellent, cosmetics, topical medications, or other substances. Until the treated knee(s) is completely dry, avoid skin-to-skin contact between other people and the treated knee(s). ( 2.2 ) Do not get Diclofenac Sodium Topical Solution in your eyes, nose or mouth ( 2.2 ). 2.1 General Dosing Instructions Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [ see Warnings and Precautions ( 5.2 ) ] For the relief of the signs and symptoms of osteoarthritis of the knee(s), the recommended dose is 40 drops per knee, 4 times a day. Apply Diclofenac Sodium Topical Solution to clean, dry skin. To avoid spillage, dispense Diclofenac Sodium Topical Solution 10 drops at a time either directly onto the knee or first into the hand and then onto the knee. Spread Diclofenac Sodium Topical Solution evenly around front, back and sides of the knee. Repeat this procedure until 40 drops have been applied and the knee is completely covered with solution. To treat the other knee, if symptomatic, repeat the procedure. Application of Diclofenac Sodium Topical Solution in an amount exceeding or less than the recommended dose has not been studied and is therefore not recommended. 2.2 Special Precautions Avoid showering/bathing for at least 30 minutes after the application of Diclofenac Sodium Topical Solution to the treated knee. Wash and dry hands after use. Do not apply Diclofenac Sodium Topical Solution to open wounds. Avoid contact of Diclofenac Sodium Topical Solution with eyes and mucous membranes. Do not apply external heat and/or occlusive dressings to treated knees. Avoid wearing clothing over the Diclofenac Sodium Topical Solution -treated knee(s) until the treated knee is dry. Protect the treated knee(s) from natural or artificial sunlight. Wait until the treated area is dry before applying sunscreen, insect repellant, lotion, moisturizer, cosmetics, or other topical medication to the same knee you have just treated with Diclofenac Sodium Topical Solution. Until the treated knee(s) is completely dry, avoid skin-to-skin contact between other people and the treated knee(s). Do not use combination therapy with Diclofenac Sodium Topical Solution and an oral NSAID unless the benefit outweighs the risk and conduct periodic laboratory evaluations.
