Drug Catalog - Product Detail
DICLOFENAC SODIUM ER OPHTH. SOL 0.001 5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16571-0101-50 | PACK PHARMACEUTICALS, LLC | 5 | 0.1% | NA |
PACKAGE FILES


Generic Name
DICLOFENAC SODIUM
Substance Name
DICLOFENAC SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
ANDA078553
Description
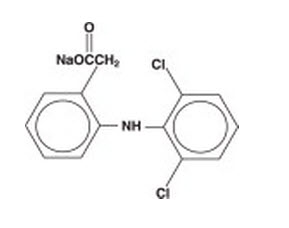
DESCRIPTION Diclofenac sodium ophthalmic solution, 0.1% is a sterile, topical, nonsteroidal, anti-inflammatory product for ophthalmic use. Diclofenac sodium is designated chemically as 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt, with an empirical formula of C 14 H 10 Cl l2 NO 2 Na. The structural formula of diclofenac sodium is: Diclofenac sodium ophthalmic solution is available as a sterile solution which contains diclofenac sodium 0.1% (1mg/mL). Inactive Ingredients: polyoxyl 35 castor oil, Boric acid, tromethamine, sorbic acid (2 mg/mL), edetate disodium (1 mg/mL), and purified water for injection. Diclofenac sodium is a faintly yellow-white to light beige, slightly hygroscopic crystalline powder. It is freely soluble in methanol, sparingly soluble in water, very slightly soluble in acetonitrile, and insoluble in chloroform and in 0.1N hydrochloric acid. Its molecular weight is 318.14. Diclofenac sodium ophthalmic solution, 0.1% is an iso osmotic solution with an osmolality of about 300 mOsmol/1000 g, buffered at approximately pH 7.2. Diclofenac sodium ophthalmic solution has a faint characteristic odor of castor oil. structure
How Supplied
HOW SUPPLIED Diclofenac sodium ophthalmic solution, 0.1% (1 mg/mL) Sterile Solution is supplied in a Low Density Polyethylene (LDPE) squeeze bottle with a LDPE dropper and a High Density Polyethylene (HDPE) closure in the following sizes: Bottles of 2.5 mL NDC 16571-101-25 Bottles of 5 mL NDC 16571-101-50 Store at 20°-25°C (68° to 77°F); [See USP Controlled Room Temperature] Protect from light. Dispense in original container. Do not use if seal on the bottle is broken. Rx only Manufactured in India for: Rising Pharmaceuticals, Inc. Allendale, NJ 07401 Distributed by: Pack Pharmaceuticals, LLC Allendale, NJ 07401
Indications & Usage
INDICATIONS AND USAGE Diclofenac sodium ophthalmic solution is indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction and for the temporary relief of pain and photophobia in patients undergoing corneal refractive surgery.
Dosage and Administration
DOSAGE AND ADMINISTRATION Cataract Surgery One drop of diclofenac sodium ophthalmic solution should be applied to the affected eye, 4 times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period. Corneal Refractive Surgery One or two drops of diclofenac sodium ophthalmic solution should be applied to the operative eye within the hour prior to corneal refractive surgery. Within 15 minutes after surgery, one or two drops should be applied to the operative eye and continued 4 times daily for up to 3 days.
