Drug Catalog - Product Detail
DICLOFENAC SODIUM DELAYED RELEASE TB 75MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16571-0201-11 | PACK PHARMACEUTICALS, LLC | 1000 | 75MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
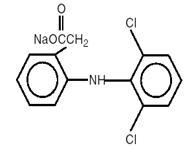
DESCRIPTION Diclofenac Sodium Delayed-release Tablets are a benzene-acetic acid derivative. Diclofenac Sodium Delayed-release Tablets are available as delayed-release (delayed-release) tablets of 25 mg or 50 mg for oral administration. The chemical name is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt. The molecular weight is 318.14. Its molecular formula is C 14 H 10 Cl 2 NNaO 2 , and it has the following structural formula The inactive ingredients in Diclofenac Sodium Delayed-release Tablets include: lactose (monohydrate), microcrystalline cellulose, croscarmellose sodium, povidone, talc, magnesium stearate, methacrylic acid copolymer, polyethylene glycol, opadry brown (Titanium dioxide, hypromellose, polyethylene glycol, iron oxide red, iron oxide yellow) and purified water. diclofenac structure
How Supplied
HOW SUPPLIED Diclofenac Sodium Delayed-release Tablets, USP, for oral administration, are available as: 25 mg : round, Light brown, enteric-coated tablets P 25 imprinted on one side in black ink and plain on the reverse side are supplied as: Bottles of 100 NDC 16571-203-10 50 mg : round, Light brown, enteric-coated tablets P 50 on one side in black ink and plain on the reverse side are supplied as: Bottles of 60 NDC 16571-202-06 Bottles of 100 NDC 16571-202-10 Bottles of 1000 NDC 16571-202-11 75 mg : round, Light brown, enteric-coated tablets P 75 imprinted on one side in black ink and plain on the reverse side are supplied as: Bottles of 60 NDC 16571-201-06 Bottles of 100 NDC 16571-201-10 Bottles of 500 NDC 16571-201-50 Bottles of 1000 NDC 16571-201-11 Store at 20°-25°C (68°-77°F) (see USP Controlled Room Temperature). Protect from moisture. Dispense in a tight, light-resistant container. Manufactured by: UNIQUE PHARMACEUTICAL LABORATORIES. (A Div. of J. B. Chemicals & Pharmaceuticals Ltd.) Mumbai 400 030, India. Distributed by: PACK Pharmaceuticals, LLC Buffalo Grove, IL 60089
Indications & Usage
INDICATIONS AND USAGE Carefully consider the potential benefits and risks of Diclofenac Sodium Delayed-release Tablets and other treatment options before deciding to use Diclofenac Sodium Delayed-release Tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). Diclofenac Sodium Delayed-release Tablets, are indicated: For relief of signs and symptoms of osteoarthritis For relief of signs and symptoms of rheumatoid arthritis For acute or long-term use in the relief of signs and symptoms of ankylosing spondylitis
Dosage and Administration
DOSAGE AND ADMINISTRATION Carefully consider the potential benefits and risks of Diclofenac Sodium Delayed-release Tablets and other treatment options before deciding to use Diclofenac Sodium Delayed-release Tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). After observing the response to initial therapy with Diclofenac Sodium Delayed-release Tablets, the dose and frequency should be adjusted to suit an individual patient’s needs. For the relief of osteoarthritis, the recommended dosage is 100-150 mg/day in divided doses (50 mg b.i.d. or t.i.d., or 75 mg b.i.d.). For the relief of rheumatoid arthritis, the recommended dosage is 150-200 mg/day in divided doses (50 mg t.i.d. or q.i.d., or 75 mg b.i.d.). For the relief of ankylosing spondylitis, the recommended dosage is 100-125 mg/day, administered as 25 mg q.i.d., with an extra 25-mg dose at bedtime if necessary. Different formulations of Diclofenac (Diclofenac sodium enteric-coated tablets; Diclofenac sodium extended-release tablets, Diclofenac potassium immediate-release tablets) are not necessarily bioequivalent even if the milligram strength is the same.
