Drug Catalog - Product Detail
DICLOFENAC SOD 3% GEL 100GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00472-1783-10 | ACTAVIS MID ATLANTIC | 100 | 3% | GEL |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
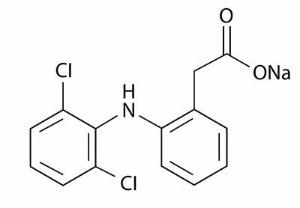
DESCRIPTION Diclofenac sodium gel, 3%, contains the active ingredient, diclofenac sodium USP, in a clear, transparent, colorless to slightly yellow gel base. Diclofenac sodium, USP is a white to slightly yellow crystalline powder. It is freely soluble in methanol, soluble in ethanol, sparingly soluble in water, slightly soluble in acetone, and partially insoluble in ether. The chemical name for diclofenac sodium is: Sodium [ o -(2,6-dichloranilino) phenyl] acetate Diclofenac sodium, USP has a molecular weight of 318.13. The CAS number is CAS-15307-79-6. The structural formula is represented below: Diclofenac sodium gel, 3% also contains benzyl alcohol, hyaluronate sodium, polyethylene glycol monomethyl ether, and purified water. 1 g of diclofenac sodium gel, 3% contains 30 mg of the active substance, diclofenac sodium, USP. 1
How Supplied
HOW SUPPLIED Available in tubes of 100 g (NDC 0472-1783-10). Each gram of gel contains 30 mg of diclofenac sodium, USP. Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from heat. Avoid freezing. †Brands listed are the trademarks of their respective owners. Distributed by: Actavis Pharma, Inc. Parsippany, NJ 07054 USA Rev. B 10/2021 3701600-6300 GW7341
Indications & Usage
INDICATIONS AND USAGE Diclofenac sodium gel, 3% is indicated for the topical treatment of actinic keratoses (AK). Sun avoidance is indicated during therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Diclofenac sodium gel, 3% is applied to lesion areas twice daily. It is to be smoothed onto the affected skin gently. The amount needed depends upon the size of the lesion site. Assure that enough diclofenac sodium gel, 3% is applied to adequately cover each lesion. Normally 0.5 g of gel is used on each 5 cm x 5 cm lesion site. The recommended duration of therapy is from 60 days to 90 days. Complete healing of the lesion(s) or optimal therapeutic effect may not be evident for up to 30 days following cessation of therapy. Lesions that do not respond to therapy should be carefully re-evaluated and management reconsidered.
