Drug Catalog - Product Detail
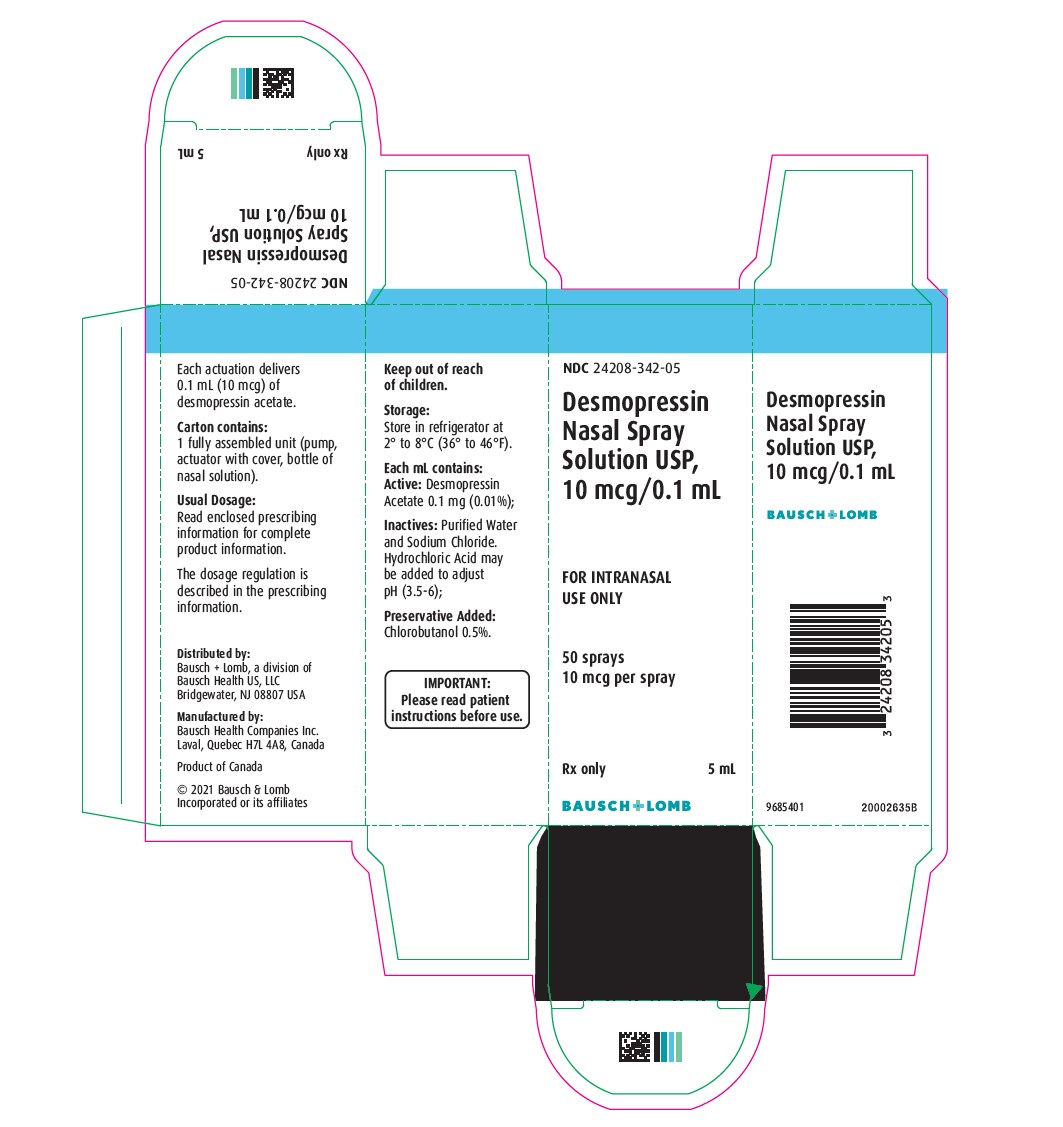
DESMOPRESSIN ACETATE NASAL SOL. SOL 0.0001 5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0342-05 | BAUSCH HEALTH US | 5 | 0.01% | SOLUTION |
PACKAGE FILES
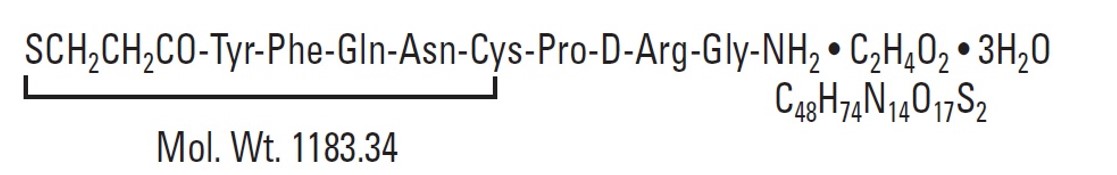


Generic Name
DESMOPRESSIN ACETATE
Substance Name
DESMOPRESSIN ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
NASAL
Application Number
ANDA074830
Description
11 DESCRIPTION Desmopressin Nasal Spray Solution, USP is a vasopressin analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows: Molecular weight: 1183.34 Empirical formula: C 46 H 64 N 14 O 12 S 2 •C 2 H 4 O 2 •3H 2 O 1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate. Desmopressin Nasal Spray Solution, USP is an aqueous solution for intranasal use. Each mL contains: ACTIVE: Desmopressin Acetate 0.1 mg (0.01%); INACTIVES: Purified Water and Sodium Chloride. Hydrochloric Acid may be added to adjust pH (3.5 - 6.0). PRESERVATIVE ADDED: Chlorobutanol 0.5%. chemstructure
How Supplied
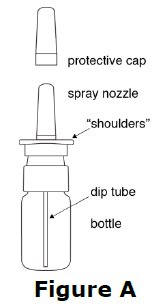
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Desmopressin Nasal Spray Solution, USP is available as a 5 mL bottle containing an aqueous solution with the spray pump delivering 50 sprays of 10 mcg (0.1 mL) (NDC 24208-342-05). 16.2 Storage and Handling Store in refrigerator at 2° to 8°C (36° to 46°F). When traveling, product will maintain stability for up to 3 weeks when stored at room temperature, 22°C (72°F). STORE BOTTLE IN UPRIGHT POSITION.
Indications & Usage
1 INDICATIONS AND USAGE Desmopressin nasal spray solution is indicated as antidiuretic replacement therapy in the management of central diabetes insipidus in adults and pediatric patients 4 years of age and older. Limitations of Use: Desmopressin nasal spray solution is not indicated for: • Treatment of nephrogenic diabetes insipidus, • Treatment of primary nocturnal enuresis [ see Warnings and Precautions (5.1) ], • Use in patients with conditions that compromise the intranasal route of administration (e.g., severe nasal congestion and blockage, nasal mucosa atrophy, severe atrophic rhinitis, recent nasal surgery such as transsphenoidal hypophysectomy) [see Warnings and Precautions (5.2) ], • Use in patients with an impaired level of consciousness, • Use in patients requiring doses less than 10 mcg or doses that are not multiples of 10 mcg [see Dosage Forms and Strengths (3)]. Desmopressin nasal spray solution is a vasopressin analog indicated as antidiuretic replacement therapy in the management of central diabetes insipidus for adults and pediatric patients 4 years of age and older ( 1 ) Limitations of Use: Desmopressin nasal spray solution is not indicated for: • Treatment of nephrogenic diabetes insipidus ( 1 ) • Treatment of primary nocturnal enuresis ( 1 , 5.1 ) • Use in patients with conditions that compromise intranasal route of administration ( 1 , 5.2 ) • Use in patients with an impaired level of consciousness ( 1 ) • Use in patients requiring doses less than 10 mcg or doses that are not multiples of 10 mcg ( 1 , 3 )
Dosage and Administration
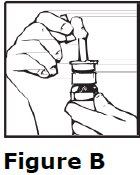
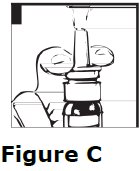
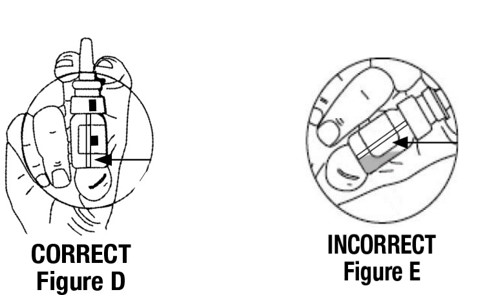
2 DOSAGE AND ADMINISTRATION • For intranasal use only ( 2.1 ) • Instruct patients to prime pump prior to use ( 2.1 ) • Adults: 10 mcg to 40 mcg daily (either as a single dose or divided into two or three daily doses) ( 2.2 ) • Pediatrics: 10 mcg once daily into one nostril up to 30 mcg once daily (or 30 mcg divided as 20 mcg during the morning and 10 mcg at night) ( 2.2 ) • See the Full Prescribing Information for recommendations for switching between desmopressin acetate formulations ( 2.3 ) 2.1 Important Administration Instructions Administer desmopressin nasal spray solution by intranasal use only. Instruct patients about appropriate fluid restriction during desmopressin nasal spray solution treatment [see Warnings and Precautions (5.1) ]. Must prime the spray pump prior to the first use. Instruct patients to: • Prime pump by pressing down on pump five times (if the spray pump is not used for one week, re-prime the pump by pressing down on the pump once). • Discard desmopressin nasal spray solution after 50 sprays since the amount delivered thereafter may be substantially less than the recommended dosage. 2.2 Recommended Dosage The use of desmopressin nasal spray solution is not indicated for patients who require less than 10 mcg doses or doses that are not multiples of 10 mcg because the spray pump can only deliver doses of 10 mcg [see Indications and Usage (1) ] . If other doses are required, use another desmopressin acetate product. Individualize the dosage of desmopressin nasal spray solution for each patient with particular attention in pediatric and elderly patients and adjust according to the diurnal pattern of response to limit nocturia and to ensure fluid intake with respect to urine output is not excessive [see Warnings and Precautions (5.1) ]. Monitor continued response to desmopressin nasal spray solution by urine volume and osmolality to ensure adequate diuresis to limit the risk of hyponatremia, and include measurements of serum sodium and plasma osmolality as needed. Adults The recommended dosage in adults is 10 mcg once daily into one nostril up to 40 mcg once daily (or 40 mcg divided into two or three daily doses). If administered more than once a day, adjust for an adequate diurnal rhythm of urine output. Pediatric Patients • For pediatric patients requiring doses less than 10 mcg, desmopressin nasal spray solution is not indicated. • For pediatric patients 4 years of age and older, the recommended starting dosage of desmopressin nasal spray solution is 10 mcg once daily into one nostril. The dose can be titrated up to 30 mcg once daily (or 30 mcg divided into two daily doses, typically with 20 mcg given in the morning and 10 mcg given at nighttime). If administered more than once a day, adjust for an adequate diurnal rhythm of urine output. Because administration of desmopressin acetate can be associated with decreased responsiveness with prolonged use, consider increasing the dosage of desmopressin nasal spray solution if patients demonstrate decreased response over a long period of time. 2.3 Switching Between Desmopressin Acetate Formulations When switching from the desmopressin acetate injection to desmopressin nasal spray solution, administer 10 times the amount of desmopressin acetate, rounding down to the nearest 10 mcg. When switching from the desmopressin acetate tablets to desmopressin nasal spray solution, individual dose titration is required because intranasal desmopressin is approximately 10 to 40 fold more potent than oral (tablet) desmopressin.
