Drug Catalog - Product Detail
DARIFENACIN HYDROBROMIDE TAB ER 24HR 7.5 MG (BASE EQUIV) 90 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 33342-0276-10 | MACLEODS PHARMACEUTICALS | 90 | 7.5MG | NA |
PACKAGE FILES



Generic Name
DARIFENACIN
Substance Name
DARIFENACIN HYDROBROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207302
Description
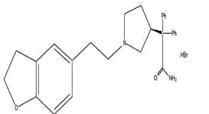
11 DESCRIPTION Darifenacin is an extended-release tablet for oral administration which contains 7.5 mg or 15 mg darifenacin as its hydrobromide salt. The active moiety, darifenacin, is a potent muscarinic receptor antagonist. Chemically, darifenacin hydrobromide is (S)-2-{1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]-3-pyrrolidinyl}-2,2-diphenylacetamide hydrobromide. The empirical formula of darifenacin hydrobromide is C 28 H 30 N 2 O 2 •HBr. The structural formula is: Darifenacin hydrobromide is a white to almost white, crystalline powder, with a molecular weight of 507.5. Darifenacin is a once-a-day extended-release tablet and contains the following inactive ingredients: dibasic calcium phosphate anhydrous, hypromellose, colloidal silicon dioxide, magnesium stearate, polyethylene glycol, talc, titanium dioxide. The 15 mg tablet also contains ferric oxide red and ferric oxide yellow. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Darifenacin extended-release tablets, 7.5 mg are white-colored, round, bi-convex, film coated and are debossed with “L48” on one side and plain on the other side. Bottle of 30 ....................................... NDC 33342-276-07 Bottle of 90 ....................................... NDC 33342-276-10 Carton of 100 tablets (10 x 10 unit-dose)….. NDC 33342-276-12 Darifenacin extended-release tablets, 15 mg are light peach-colored, round, bi-convex, film coated and are debossed with “L49” on one side and plain on the other side. Bottle of 30 ................................ NDC 33342-277-07 Bottle of 90 ................................ NDC 33342-277-10 Carton of 100 tablets (10 x 10 unit-dose)… NDC 33342-277-12 Storage Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light. Keep this and all drugs out of the reach of children.
Indications & Usage
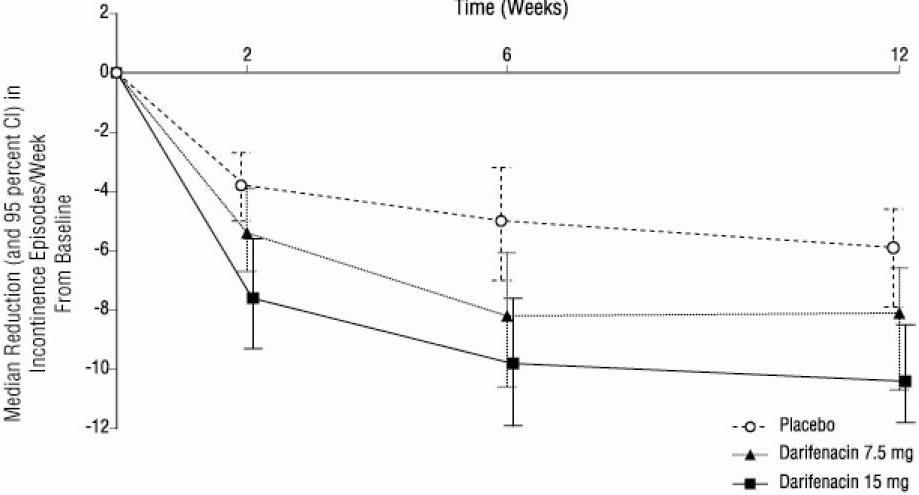
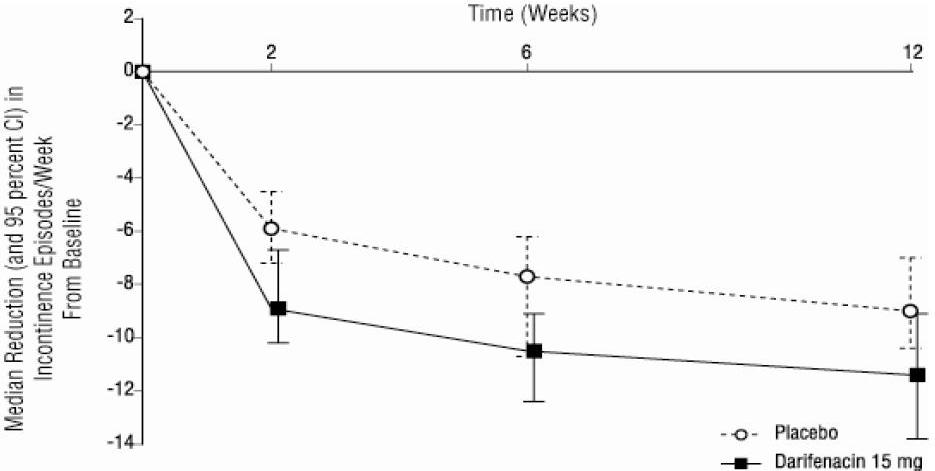
1 INDICATIONS & USAGE Darifenacin is indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency. Darifenacin extended-release tablet is a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency. ( 1 )
Dosage and Administration
2 DOSAGE & ADMINISTRATION The recommended starting dose of darifenacin is 7.5 mg orally once daily. Based upon individual response, the dose may be increased to 15 mg once daily, as early as two weeks after starting therapy. Darifenacin should be taken orally once daily with water. Darifenacin may be taken with or without food, and should be swallowed whole and not chewed, divided or crushed. For patients with moderate hepatic impairment (Child-Pugh B) or when co-administered with potent CYP3A4 inhibitors (for example, ketoconazole, itraconazole, ritonavir, nelfinavir, clarithromycin and nefazadone), the daily dose of darifenacin should not exceed 7.5 mg. Darifenacin is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) [see Warnings & Precautions ( 5.6 ), Drug Interactions ( 7.1 ), Use in Specific Populations ( 8.6 ) and Clinical Pharmacology ( 12.3 )]. The recommended starting dose of darifenacin extended-release tablets is 7.5 mg once daily. Based upon individual response, the dose may be increased to 15 mg once daily, as early as two weeks after starting therapy ( 2 ) The daily dose of darifenacin should not exceed 7.5 mg in the following patients: • Patients with moderate hepatic impairment (Child- Pugh B) ( 2 , 8.6 ) • Patients taking potent CYP3A4 inhibitors ( 2 , 7.1 ) Darifenacin is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) ( 2 , 8.6 ) Darifenacin may be taken with or without food. The tablet should be swallowed whole with water and not chewed, divided or crushed ( 2 )
