Drug Catalog - Product Detail
CYCLOPHOSPHAMIDE 25MG 100 CP
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0516-07 | CIPLA USA | 100 | 25MG | CAPSULE |
PACKAGE FILES

Generic Name
CYCLOPHOSPHAMIDE
Substance Name
CYCLOPHOSPHAMIDE ANHYDROUS
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA211608
Description
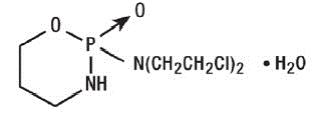
11 DESCRIPTION Cyclophosphamide is a synthetic antineoplastic drug chemically related to the nitrogen mustards. The chemical name for cyclophosphamide is 2-[bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide monohydrate, and has the following structural formula: Cyclophosphamide has a molecular formula of C 7 H 15 Cl 2 N 2 O 2 P•H 2 O and a molecular weight of 279.10. Cyclophosphamide is soluble in water and alcohol. Each capsule for oral use contains 25 mg or 50 mg cyclophosphamide (monohydrate, USP) and the following inactive ingredients: pregelatinized starch and sodium stearyl fumarate. Each gelatin capsule shell contains FD&C Blue #1, FD&C Red #3, gelatin and titanium dioxide. In addition to the ingredients listed above, each capsule contains black ink. Black ink contains, black iron oxide, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, dehydrated alcohol, potassium hydroxide, purified water and shellac. Image
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Cyclophosphamide Capsules 25 mg, s upplied as white to off white blend filled in hard gelatin capsule size 3, cap opaque white imprinted with "Cipla" in black ink and body opaque blue imprinted with "516 25 MG" with black ink NDC 69097-516-07: 100's Bottle with child-resistant closure 50 mg, s upplied as white to off white blend filled in hard gelatin capsule size 3, cap opaque white imprinted with "Cipla" in black ink and body opaque blue imprinted with "516 25 MG" with black ink NDC 69097-517-07: 100's Bottle with child-resistant closure Storage This package is child-resistant. Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature.] Cyclophosphamide is an antineoplastic product. Follow special handling and disposal procedures. 1
Indications & Usage
1 INDICATIONS AND USAGE Cyclophosphamide capsules is an alkylating drug indicated for treatment of: Malignant Diseases: malignant lymphomas: Hodgkin's disease, lymphocytic lymphoma, mixed-cell type lymphoma, histiocytic lymphoma, Burkitt's lymphoma; multiple myeloma, leukemias, mycosis fungoides, neuroblastoma, adenocarcinoma of ovary, retinoblastoma, breast carcinoma (1.1) Minimal Change Nephrotic Syndrome in Pediatric Patients: biopsy proven minimal change nephrotic syndrome in pediatric patients who failed to adequately respond to or are unable to tolerate adrenocorticosteroid therapy (1.2) Limitations of Use: The safety and effectiveness for the treatment of nephrotic syndrome in adults or other renal disease has not been established. 1.1 Malignant Diseases Cyclophosphamide capsules are indicated for the treatment of: malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin's disease, lymphocytic lymphoma (nodular or diffuse), mixed-cell type lymphoma, histiocytic lymphoma, Burkitt's lymphoma multiple myeloma leukemias: chronic lymphocytic leukemia, chronic granulocytic leukemia (it is usually ineffective in acute blastic crisis), acute myelogenous and monocytic leukemia, acute lymphoblastic (stem-cell) leukemia (cyclophosphamide given during remission is effective in prolonging its duration) mycosis fungoides (advanced disease) neuroblastoma (disseminated disease) adenocarcinoma of the ovary retinoblastoma carcinoma of the breast Cyclophosphamide, although effective alone in susceptible malignancies, is more frequently used concurrently or sequentially with other antineoplastic drugs. 1.2 Minimal Change Nephrotic Syndrome in Pediatric Patients Cyclophosphamide capsules is indicated for the treatment of biopsy proven minimal change nephrotic syndrome in pediatric patients who failed to adequately respond to or are unable to tolerate adrenocorticosteroid therapy. Limitations of Use: The safety and effectiveness of cyclophosphamide capsules for the treatment of nephrotic syndrome in adults or other renal disease has not been established.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION During or immediately after the administration, adequate amounts of fluid should be ingested or infused to force diuresis in order to reduce the risk of urinary tract toxicity. Therefore, cyclophosphamide should be administered in the morning. During or immediately after the administration, administer adequate amounts of fluid to reduce the risk of urinary tract toxicity (2.1). Malignant Diseases: Adult and Pediatric Patients (2.2) Oral: Usually 1 mg per kg per day to 5 mg per kg orally once daily for both initial and maintenance dosing. Minimal Change Nephrotic Syndrome in Pediatric Patients (2.3) Recommended oral dose: 2 mg per kg once daily for 8 to 12 weeks (maximum cumulative dose 168 mg per kg). Treatment beyond 90 days increases the probability of sterility in males. 2.1 Hydration and Important Administration Instructions During or immediately after the administration of cyclophosphamide capsules, adequate amounts of fluid should be ingested or infused to force diuresis in order to reduce the risk of urinary tract toxicity. Therefore, cyclophosphamide capsules should be taken in the morning. Cyclophosphamide capsules should be swallowed whole. The capsules should not be opened, chewed, or crushed. Cyclophosphamide capsules is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1 Exposure to broken capsules should be avoided. If contact with broken capsules occurs, wash hands immediately and thoroughly. 2.2 Recommended Dosage for Malignant Disease Adults and Pediatric Patients The recommended dosage of cyclophosphamide capsule is in the range of 1 mg per kg to 5 mg per kg orally once daily for both initial and maintenance dosing. Other regimens of intravenous and oral cyclophosphamide have been reported. Dosages should be adjusted based on evidence of antitumor activity, myelosuppression, or other severe adverse reactions [see Warnings and Precautions (5)] . When cyclophosphamide is included in combined cytotoxic regimens, it may be necessary to reduce the dose of cyclophosphamide, as well as that of the other drugs. 2.3 Recommended Dosage for Minimal Change Nephrotic Syndrome in Pediatric Patients The recommended dosage of cyclophosphamide capsule is 2 mg per kg orally once daily for 8 to 12 weeks (maximum cumulative dose 168 mg per kg). Treatment beyond 90 days increases the probability of sterility in males [see Use in Specific Populations (8.4)] .
