Drug Catalog - Product Detail
CYCLOBENZAPRINE HCL TB 5MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 59746-0211-10 | JUBILANT CADISTA | 1000 | 5MG | TABLET |
PACKAGE FILES


Generic Name
CYCLOBENZAPRINE HYDROCHLORIDE
Substance Name
CYCLOBENZAPRINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077563
Description
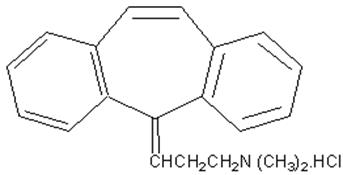
DESCRIPTION Cyclobenzaprine hydrochloride, USP is a white, crystalline tricyclic amine salt with the empirical formula C 20 H 21 N•HCl and a molecular weight of 311.9. It has a melting point of 217º C, and a pKa of 8.47 at 25º C. It is freely soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base separates. Cyclobenzaprine Hydrochloride is designated chemically as 3-(5H-dibenzo [a,d] cyclohepten-5-ylidene)-N, N-dimethyl-1-propanamine hydrochloride, and has the following structural formula: Cyclobenzaprine hydrochloride tablets USP, for oral administration, are available in the following strengths: 5 mg, 7.5 mg and 10 mg. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide. In addition, the 5 mg tablet contain FD&C yellow # 6, and both the 5 mg and 10 mg tablets contain iron oxide yellow.
How Supplied
HOW SUPPLIED Cyclobenzaprine Hydrochloride Tablets, USP are available in the following strengths and package sizes: 5 mg (Orange, round, film-coated tablets, debossed with “TL 211” on one side and plain on the other side) Bottles of 100’s NDC 59746-211-06 Bottles of 1000’s NDC 59746-211-10 7.5 mg (White, round, film coated tablets, debossed with “C 735” on one side and plain on the other side) Bottles of 30’s NDC 59746-735-30 Bottles of 100’s NDC 59746-735-01 Bottles of 1000’s NDC 59746-735-10 10 mg (Yellow, round, film-coated tablets, debossed with “TL 177” on one side and plain on the other side) Bottles of 100’s NDC 59746-177-06 Bottles of 1000’s NDC 59746-177-10 Store at 20º to 25°C (68º to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Indications & Usage
INDICATIONS AND USAGE Cyclobenzaprine hydrochloride tablets, USP are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, limitation of motion, and restriction in activities of daily living. Cyclobenzaprine hydrochloride tablets should be used only for short periods (up to 2 or 3 weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted. Cyclobenzaprine hydrochloride tablets have not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease, or in children with cerebral palsy.
Dosage and Administration
DOSAGE AND ADMINISTRATION For most patients, the recommended dose of cyclobenzaprine hydrochloride tablets is 5 mg three times a day. Based on individual patient response, the dose may be increased to 10 mg three times a day. Use of cyclobenzaprine hydrochloride tablets for periods longer than 2 or 3 weeks is not recommended (see INDICATIONS AND USAGE). Less frequent dosing should be considered for hepatically impaired or elderly patients (see PRECAUTIONS : Impaired Hepatic Function , and Use in the Elderly ).
