Drug Catalog - Product Detail
CYCLOBENZAPRINE HCL TAB 7.5MG 60CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 53217-0170-60 | AIDAREX PHARMACEUTICALS | 60 | 7.5MG | NA |
PACKAGE FILES
Generic Name
Substance Name
Product Type
Route
Application Number
Description
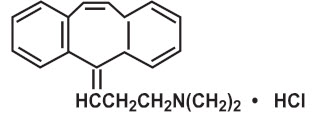
DESCRIPTION Cyclobenzaprine hydrochloride, USP is a white to off-white crystalline powder with the molecular formula C 20 H 21 N•HCl and a molecular weight of 311.9. It has a melting point of 217° C, and a pK a of 8.47 at 25° C. It is freely soluble in water, in alcohol and in methanol, sparingly soluble in isopropanol, slightly soluble in chloroform and in methylene chloride and insoluble in hydrocarbons. If aqueous solutions are made alkaline, the free base separates. Cyclobenzaprine HCl is designated chemically as 3-(5H-dibenzo[a,d] cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride, and has the following structural formula: Cyclobenzaprine hydrochloride tablets, USP are supplied as 7.5 mg tablets for oral administration. Cyclobenzaprine hydrochloride 7.5 mg tablets contain the following inactive ingredients: corn starch, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, pregelatinized starch, talc and titanium dioxide. The structural formula of cyclobenzaprine hydrochloride.
How Supplied
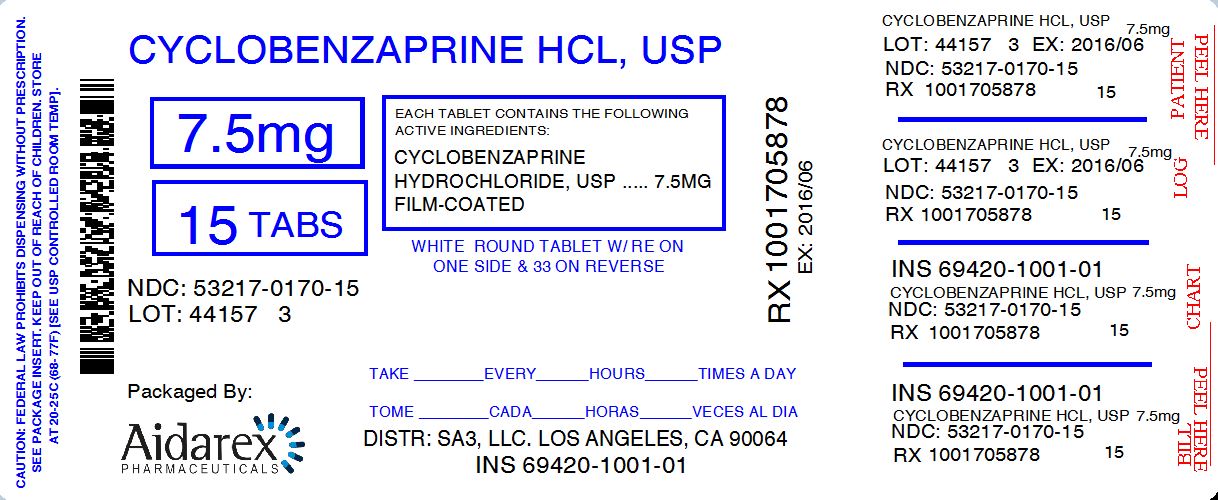
HOW SUPPLIED Cyclobenzaprine hydrochloride tablets, USP are available in 7.5 mg dosage strength. The dosage strength is supplied as follows: The 7.5 mg tablets are white, round shaped, biconvex, film-coated tablets debossed with ‘RE’ on one side and ‘33’ on other side. 15 TABLET in a BOTTLE, PLASTIC (53217-170-15) 30 TABLET in a BOTTLE, PLASTIC (53217-170-30) 60 TABLET in a BOTTLE, PLASTIC (53217-170-60) 90 TABLET in a BOTTLE, PLASTIC (53217-170-90) Store between 20 - 25° C (68 - 77° F). [See USP Controlled Room Temperature]. To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . Distributed by: SA3, LLC. Los Angeles, CA 90064 February 2015 FDA-01 Repackage By: Aidarex Pharmaceuticals, LLC Corona CA.
Indications & Usage
INDICATIONS AND USAGE Cyclobenzaprine hydrochloride tablets, USP are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, limitation of motion, and restriction in activities of daily living. Cyclobenzaprine hydrochloride tablets, USP should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted. Cyclobenzaprine hydrochloride tablets, USP have not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease, or in children with cerebral palsy.
Dosage and Administration
DOSAGE AND ADMINISTRATION For most patients, the recommended dose of cyclobenzaprine hydrochloride tablets is 5 mg three times a day. Based on individual patient response, the dose may be increased to either 7.5 or 10 mg three times a day. Use of cyclobenzaprine hydrochloride tablets for periods longer than two or three weeks is not recommended. (see INDICATIONS AND USAGE ). Less frequent dosing should be considered for hepatically impaired or elderly patients (see PRECAUTIONS , Impaired Hepatic Function , and Use in the Elderly ).
