Drug Catalog - Product Detail
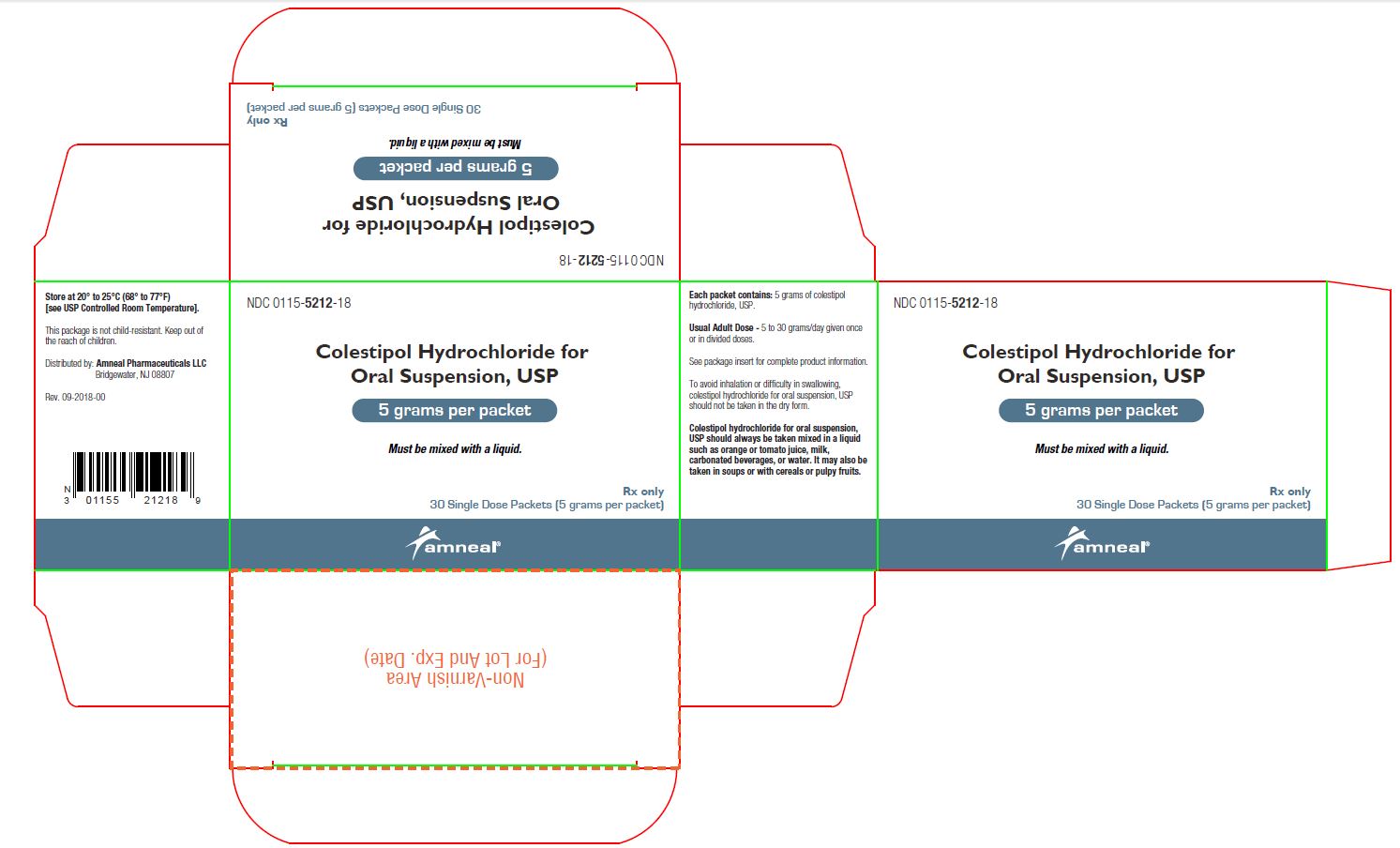
COLESTIPOL HCL FOR ORAL SUSPENSION, USP GRAN 5G/PCKT. 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-5212-29 | AMNEAL PHARMACEUTICALS | 90 | 5GM | NA |
PACKAGE FILES

Generic Name
COLESTIPOL HYDROCHLORIDE
Substance Name
COLESTIPOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077277
Description
DESCRIPTION Colestipol hydrochloride for oral suspension, USP contains colestipol hydrochloride USP, which is a lipid lowering agent for oral use. Colestipol hydrochloride is an insoluble, high molecular weight basic anion-exchange copolymer of diethylenetriamine and 1-chloro-2, 3-epoxypropane, with approximately 1 out of 5 amine nitrogens protonated (chloride form). It is a light yellow water-insoluble resin which is hygroscopic and swells when suspended in water or aqueous fluids. Colestipol hydrochloride for oral suspension, USP is tasteless and odorless. The inactive ingredient is silicon dioxide. One dose (1 packet or 1 level scoopful) of colestipol hydrochloride for oral suspension, USP contains 5 grams of colestipol hydrochloride, USP.
How Supplied
HOW SUPPLIED Colestipol hydrochloride for oral suspension, USP is available as follows: Carton of 30 foil packets: NDC 0115-5212-18 Carton of 90 foil packets: NDC 0115-5212-29 Bottle of 500 grams with scoop: NDC 0115-5213-02 Each packet or level scoop supplies 5 grams of colestipol hydrochloride, USP for oral suspension. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Since no drug is innocuous, strict attention should be paid to the indications and contraindications, particularly when selecting drugs for chronic long-term use. Colestipol hydrochloride for oral suspension is indicated as adjunctive therapy to diet for the reduction of elevated serum total and low-density lipoprotein (LDL) cholesterol in patients with primary hypercholesterolemia (elevated low density lipoproteins [LDL] cholesterol) who do not respond adequately to diet. Generally, colestipol hydrochloride for oral suspension has no clinically significant effect on serum triglycerides, but with its use triglyceride levels may be raised in some patients. Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Treatment should begin and continue with dietary therapy (see NCEP guidelines). A minimum of six months of intensive dietary therapy and counseling should be carried out prior to initiation of drug therapy. Shorter periods may be considered in patients with severe elevations of LDL-C or with definite CHD. According to the NCEP guidelines, the goal of treatment is to lower LDL-C, and LDL-C is to be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the Total-C be used to monitor therapy. The NCEP treatment guidelines are shown below. LDL-Cholesterol mg/dL (mmol/L) Definite Atherosclerotic Disease* Two or More Other Risk Factors** Initiation Level Goal No No ≥ 190 (≥ 4.9) < 160 (< 4.1) No Yes ≥ 160 (≥ 4.1) < 130 (< 3.4) Yes Yes or No ≥ 130 (≥ 3.4) ≤ 100 (≤ 2.6) * Coronary heart disease or peripheral vascular disease (including symptomatic carotid artery disease). ** Other risk factors for coronary heart disease (CHD) include: age (males: ≥ 45 years; females: ≥ 55 years or premature menopause without estrogen replacement therapy); family history of premature CHD; current cigarette smoking; hypertension; confirmed HDL-C < 35 mg/dL (0.91 mmol/L); and diabetes mellitus. Subtract one risk factor if HDL-C is ≥ 60 mg/dL (1.6 mmol/L).
Dosage and Administration
DOSAGE AND ADMINISTRATION One dose (1 packet or 1 level scoopful) of colestipol hydrochloride for oral suspension contains 5 grams of colestipol hydrochloride. The recommended daily adult dose is one to six packets or level scoopfuls given once or in divided doses. Treatment should be started with one dose once or twice daily with an increment of one dose/day at one- or two-month intervals. Appropriate use of lipid profiles as per NCEP guidelines including LDL-cholesterol and triglycerides is advised so that optimal, but not excessive doses are used to obtain the desired therapeutic effect on LDL-cholesterol level. If the desired therapeutic effect is not obtained at one to six doses/day with good compliance and acceptable side effects, combined therapy or alternate treatment should be considered. To avoid accidental inhalation or esophageal distress, colestipol hydrochloride for oral suspension should not be taken in its dry form. Colestipol hydrochloride for oral suspension should always be mixed with water or other fluids before ingesting. Patients should take other drugs at least one hour before or four hours after colestipol hydrochloride for oral suspension to minimize possible interference with their absorption ( see PRECAUTIONS, Drug Interactions ). The scoop accompanying this product is not interchangeable with other scoops. Before Colestipol Hydrochloride for Oral Suspension Administration Define the type of hyperlipoproteinemia, as described in NCEP guidelines. Institute a trial of diet and weight reduction. Establish baseline serum total and LDL-cholesterol and triglyceride levels. During Colestipol Hydrochloride for Oral Suspension Administration The patient should be carefully monitored clinically, including serum cholesterol and triglyceride levels. Periodic determinations of serum cholesterol levels as outlined in the NCEP guidelines should be done to confirm a favorable initial and longer-term response. Failure of total or LDL-cholesterol to fall within the desired range should lead one to first examine dietary and drug compliance. If these are deemed acceptable, combined therapy or alternate treatment should be considered. Significant rise in triglyceride level should be considered as indication for dose reduction, drug discontinuation, or combined or alternate therapy. Mixing and Administration Guide Colestipol hydrochloride for oral suspension should always be mixed in a liquid such as water or the beverage of your choice. It may also be taken in soups or with cereals or pulpy fruits. Colestipol hydrochloride for oral suspension should never be taken in its dry form . With Beverages Add the prescribed amount of colestipol hydrochloride for oral suspension to a glassful (three ounces or more) of water or the beverage of your choice. A heavy or pulpy juice may minimize complaints relative to consistency. Stir the mixture until the medication is completely mixed. (Colestipol hydrochloride for oral suspension will not dissolve in the liquid.) Colestipol hydrochloride for oral suspension may also be mixed with carbonated beverages, slowly stirred in a large glass; however, this mixture may be associated with GI complaints. Rinse the glass with a small amount of additional beverage to make sure all the medication is taken. With cereals, soups, and fruits Colestipol hydrochloride for oral suspension may be taken mixed with milk in hot or regular breakfast cereals, or even mixed in soups that have a high fluid content. It may also be added to fruits that are pulpy such as crushed pineapple, pears, peaches, or fruit cocktail.
