Drug Catalog - Product Detail
CLOTRIMAZOLE CREAM USP CRM 0.01 45GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0181-47 | GLENMARK PHARMACEUTICALS | 45 | 1% | CREAM |
PACKAGE FILES


Generic Name
CLOTRIMAZOLE
Substance Name
CLOTRIMAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA090219
Description
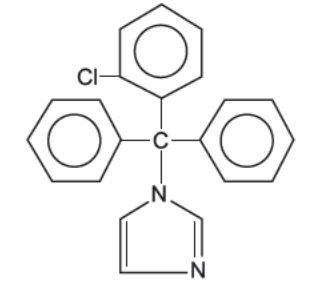
DESCRIPTION Clotrimazole cream USP, 1% contains clotrimazole, a synthetic antifungal agent having the chemical name {1-(o-Chloro-α, α-diphenylbenzyl)imidazole}; the molecular formula C 22 H 17 ClN 2 ; a molecular weight of 344.84; and the structural formula: Clotrimazole USP is an odorless, white crystalline substance. It is practically insoluble in water, sparingly soluble in ether and very soluble in polyethylene glycol 400, ethanol and chloroform. Each gram of clotrimazole cream USP contains 10 mg clotrimazole USP, dispersed in a vanishing cream base of sorbitan monostearate, polysorbate 60, cetyl esters wax, cetostearyl alcohol, octyldodecanol, purified water, and benzyl alcohol (1%) as preservative. structural formula
How Supplied
HOW SUPPLIED Clotrimazole cream USP, 1% is supplied in 15, 30, 45 and (2 x 45) gram tubes. NDC 68462-181-17 (15 g) NDC 68462-181-35 (30 g) NDC 68462-181-47 (45 g) NDC 68462-181-48 (2 x 45 g)
Indications & Usage
INDICATIONS AND USAGE Clotrimazole cream USP is indicated for the topical treatment of candidiasis due to Candida albicans and tinea versicolor due to Malassezia furfur. Clotrimazole is also available as a nonprescription item which is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum,Trichophyton mentagrophytes , Epidermophyton floccosum , and Microsporum canis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Gently massage sufficient clotrimazole cream into the affected and surrounding skin areas twice a day, in the morning and evening. Clinical improvement, with relief of pruritis, usually occurs within the first week of treatment with clotrimazole cream. If the patient shows no clinical improvement after four weeks of treatment with clotrimazole cream, the diagnosis should be reviewed.
