Drug Catalog - Product Detail
CLOPIDOGREL BI SULFATE TB 75MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0357-90 | AUROBINDO PHARMA | 90 | 75MG | TABLET |
PACKAGE FILES

Generic Name
CLOPIDOGREL BISULFATE
Substance Name
CLOPIDOGREL BISULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090540
Description
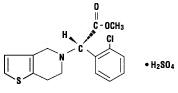
11 DESCRIPTION Clopidogrel bisulfate is a thienopyridine class inhibitor of P2Y 12 ADP platelet receptors. Chemically it is methyl (+)-( S )-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4 H )-acetate sulfate (1:1). The molecular formula of clopidogrel bisulfate is C 16 H 16 ClNO 2 S•H 2 SO 4 and its molecular weight is 419.9. The structural formula is as follows: Clopidogrel bisulfate USP is a white to off-white powder. It is practically insoluble in water at neutral pH but freely soluble at pH 1. It also dissolves freely in methanol, dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether. It has a specific optical rotation of about +56°. Clopidogrel tablets, USP for oral administration are provided as pink colored, round, biconvex, beveled edge, debossed, film-coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base. Each film-coated tablet contains crospovidone, hydrogenated castor oil, hydroxypropyl cellulose low substituted, hypromellose 15cP, iron oxide red, lactose monohydrate, mannitol, microcrystalline cellulose, polyethylene glycol 6000, titanium dioxide, and triacetin as inactive ingredients. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Clopidogrel Tablets USP, 75 mg are pink colored, round, biconvex, beveled edge, film-coated tablets debossed with ‘E’ on one side and ‘34’ on the other side. Bottles of 30 NDC 65862-357-30 Bottles of 90 NDC 65862-357-90 Bottles of 100 NDC 65862-357-01 Bottles of 500 NDC 65862-357-05 Bottles of 1,000 NDC 65862-357-99 10 x 10 Unit-dose Tablets NDC 65862-357-10 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from moisture. Preserve in well-closed containers.
Indications & Usage
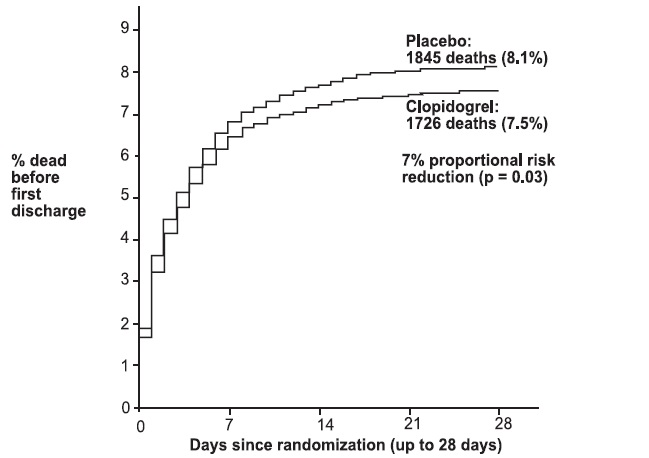
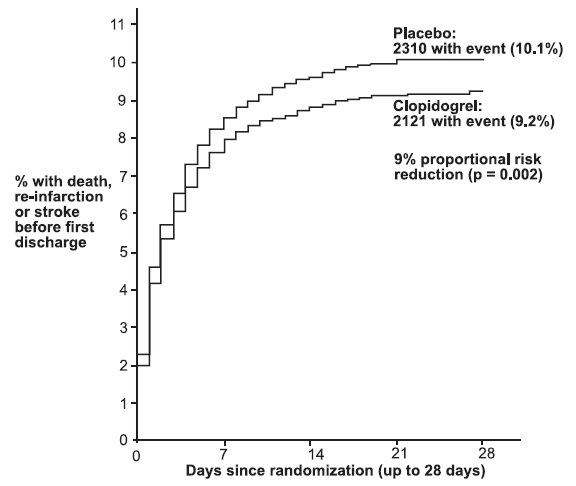
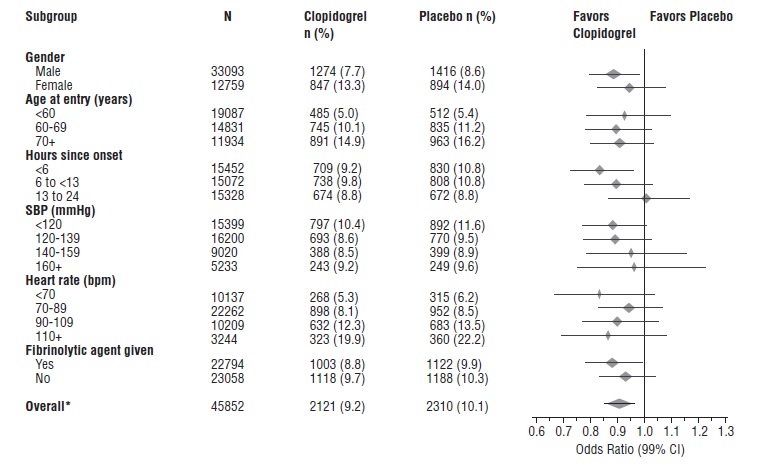
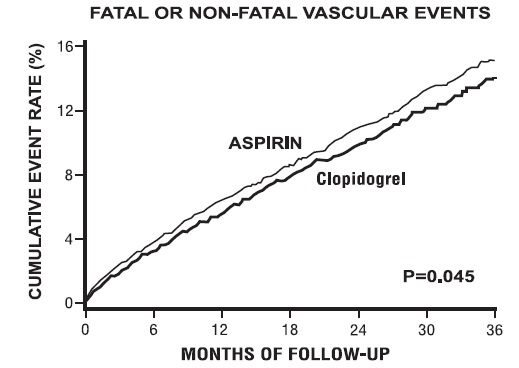
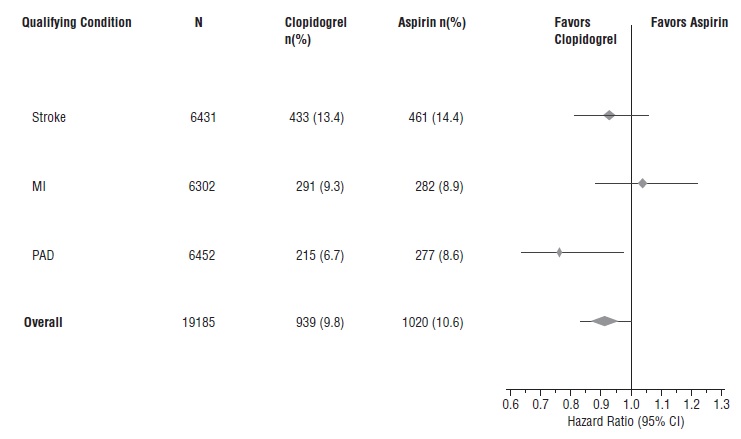
1 INDICATIONS AND USAGE Clopidogrel tablets are a P2Y 12 platelet inhibitor indicated for: Acute coronary syndrome – For patients with non–ST-segment elevation ACS (unstable angina [UA]/non–ST-elevation myocardial infarction [NSTEMI]), clopidogrel tablets have been shown to reduce the rate of myocardial infarction (MI) and stroke. (1.1) – For patients with ST-elevation myocardial infarction (STEMI), clopidogrel tablets have been shown to reduce the rate of MI and stroke. (1.1) Recent MI, recent stroke, or established peripheral arterial disease. Clopidogrel tablets have been shown to reduce the rate of MI and stroke. (1.2) 1.1 Acute Coronary Syndrome (ACS) Clopidogrel tablets are indicated to reduce the rate of myocardial infarction (MI) and stroke in patients with non–ST-segment elevation ACS (unstable angina [UA]/non–ST-elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those who are to be managed with coronary revascularization. Clopidogrel tablets should be administered in conjunction with aspirin. Clopidogrel tablets are indicated to reduce the rate of myocardial infarction and stroke in patients with acute ST-elevation myocardial infarction (STEMI) who are to be managed medically. Clopidogrel tablets should be administered in conjunction with aspirin. 1.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease In patients with established peripheral arterial disease or with a history of recent myocardial infarction (MI) or recent stroke clopidogrel tablets are indicated to reduce the rate of MI and stroke.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Acute coronary syndrome ( 2.1 ) – Initiate clopidogrel tablets with a single 300 mg oral loading dose and then continue at 75 mg once daily. – Initiating clopidogrel tablets without a loading dose will delay establishment of an antiplatelet effect by several days. Recent MI, recent stroke, or established peripheral arterial disease: 75 mg once daily orally without a loading dose. ( 2.2 ) 2.1 Acute Coronary Syndrome In patients who need an antiplatelet effect within hours, initiate clopidogrel tablets with a single 300 mg oral loading dose and then continue at 75 mg once daily. Initiating clopidogrel tablets without a loading dose will delay establishment of an antiplatelet effect by several days [see Clinical Pharmacology (12.3) and Clinical Studies (14.1) ] . 2.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease 75 mg once daily orally without a loading dose [see Clinical Pharmacology (12.3) and Clinical Studies (14.2) ] .
