Drug Catalog - Product Detail
CLONIDINE TRANSDERMAL SYSTEM 0.2MG/DAY PATCH 0.2MG/DAY 4 PACK
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-3509-04 | ACTAVIS PHARMA | 4 | 0.2MG/24HR | TRANSDERMAL SYSTEM |
PACKAGE FILES





Generic Name
CLONIDINE
Substance Name
CLONIDINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TRANSDERMAL
Application Number
ANDA090873
Description
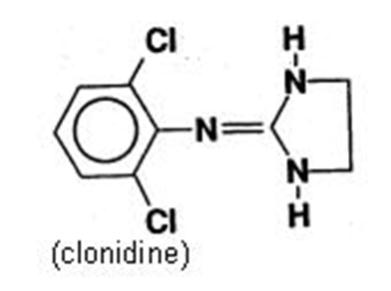
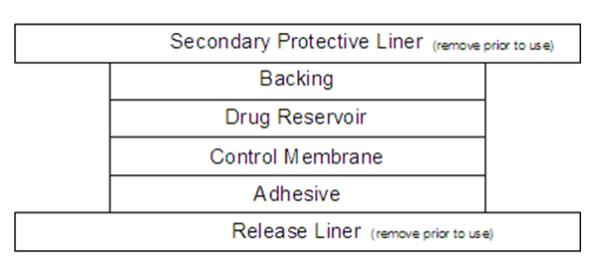
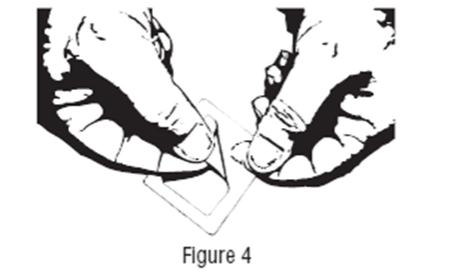
DESCRIPTION Clonidine Transdermal System, USP is a transdermal system providing continuous systemic delivery of clonidine for 7 days at an approximately constant rate. Clonidine is a centrally acting alpha-agonist hypotensive agent. It is an imidazoline derivative with the chemical name 2, 6-dichloro-N-2-imidazolidinylidenebenzenamine and has the following chemical structure: Meets USP Drug Release Test 4. The chemical structure for Clonidine. System Structure and Components Clonidine Transdermal System, USP is a multi-layered film, 0.2 mm thick, containing clonidine as the active agent. The system areas are 4.1 cm 2 (0.1 mg per day for one week), 8.2 cm 2 (0.2 mg per day for one week) and 12.3 cm 2 (0.3 mg per day for one week) and the amount of drug released is directly proportional to the area (see Release Rate Concept ). The composition per unit area is the same for all three doses. Proceeding from the visible surface towards the surface attached to the skin, there are four consecutive layers: 1) a backing layer of pigmented polyethylene/aluminum/polyester film; 2) a drug reservoir of clonidine, acrylic adhesive; 3) an ethylene vinyl acetate membrane that controls the rate of delivery of clonidine from the system to the skin surface; 4) an adhesive formulation of clonidine and acrylic adhesive. Prior to use, a translucent to clear secondary protective liner card that rests over the pigmented film is removed; also, a protective release liner of silicone coated polyester film that covers the adhesive layer is removed. Cross Section of the System: Cross Section of the System: Release Rate Concept Clonidine transdermal system is programmed to release clonidine at an approximately constant rate for 7 days. The energy for drug release is derived from the concentration gradient existing between a saturated solution of drug in the system and the much lower concentration prevailing in the skin. Clonidine flows in the direction of the lower concentration at a constant rate, limited by the rate-controlling membrane, so long as a saturated solution is maintained in the drug reservoir. Following system application to intact skin, clonidine in the adhesive layer saturates the skin site below the system. Clonidine from the drug reservoir then begins to flow through the rate-controlling membrane and the adhesive layer of the system into the systemic circulation via the capillaries beneath the skin. Therapeutic plasma clonidine levels are achieved 2 to 3 days after initial application of clonidine transdermal system. The 4.1, 8.2, and 12.3 cm 2 systems deliver 0.1, 0.2, and 0.3 mg of clonidine per day, respectively. To ensure constant release of drug for 7 days, the total drug content of the system is higher than the total amount of drug delivered. Application of a new system to a fresh skin site at weekly intervals continuously maintains therapeutic plasma concentrations of clonidine. If the clonidine transdermal system is removed and not replaced with a new system, therapeutic plasma clonidine levels will persist for about 8 hours and then decline slowly over several days. Over this time period, blood pressure returns gradually to pretreatment levels.
How Supplied
HOW SUPPLIED Clonidine transdermal systems USP, 0.1 mg per day for one week, 0.2 mg per day for one week, and 0.3 mg per day for one week are supplied as 4 pouched systems and 4 adhesive covers per carton. Each system contains 1.97 mg, 3.94 mg, or 5.90 mg clonidine. See chart below. Programmed Delivery Clonidine in vivo Per Day Over 1 Week Clonidine Content Size Code Clonidine Transdermal System, USP (clonidine) NDC 0591-3508-04 0.1 mg 1.97 mg 4.1 cm 2 WPI 3508 Clonidine Transdermal System, USP (clonidine) NDC 0591-3509-04 0.2 mg 3.94 mg 8.2 cm 2 WPI 3509 Clonidine Transdermal System, USP (clonidine) NDC 0591-3510-04 0.3 mg 5.90 mg 12.3 cm 2 WPI 3510
Indications & Usage
INDICATIONS AND USAGE Clonidine transdermal system is indicated in the treatment of hypertension. It may be employed alone or concomitantly with other antihypertensive agents.
Dosage and Administration
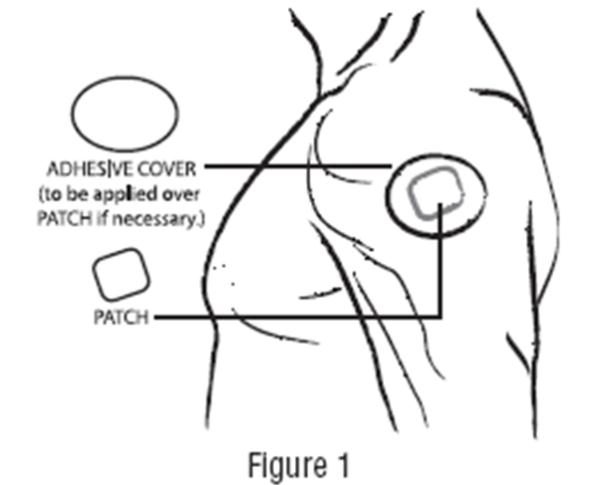
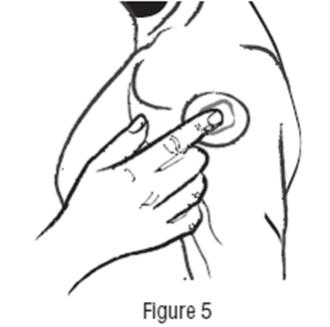
DOSAGE AND ADMINISTRATION Apply clonidine transdermal system once every 7 days to a hairless area of intact skin on the upper outer arm or chest. Each new application of clonidine transdermal system should be on a different skin site from the previous location. If the system loosens during 7-day wearing, the adhesive cover should be applied directly over the system to ensure good adhesion. There have been rare reports of the need for patch changes prior to 7 days to maintain blood pressure control. To initiate therapy, clonidine transdermal system dosage should be titrated according to individual therapeutic requirements, starting with clonidine transdermal system, 0.1 mg per day for one week. If after one or two weeks the desired reduction in blood pressure is not achieved, increase the dosage by adding another clonidine transdermal system, 0.1 mg per day for one week or changing to a larger system. An increase in dosage above two clonidine transdermal systems, 0.3 mg per day for one week is usually not associated with additional efficacy. When substituting clonidine transdermal system for oral clonidine or for other antihypertensive drugs, physicians should be aware that the antihypertensive effect of clonidine transdermal system may not commence until 2 to 3 days after initial application. Therefore, gradual reduction of prior drug dosage is advised. Some or all previous antihypertensive treatment may have to be continued, particularly in patients with more severe forms of hypertension. Renal Impairment Patients with renal impairment may benefit from a lower initial dose. Patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
