Drug Catalog - Product Detail
CLONIDINE HCL TAB 0.2MG 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 52817-0181-10 | TRUPHARMA | 100 | 0.2MG | TABLET |
PACKAGE FILES


Generic Name
CLONIDINE HYDROCHLORIDE
Substance Name
CLONIDINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA070923
Description
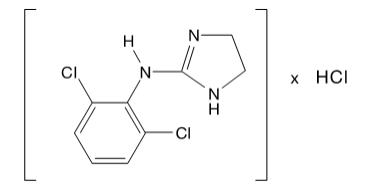
DESCRIPTION Clonidine hydrochloride is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg and 0.3 mg. The 0.1 mg tablet is equivalent to 0.087 mg of the free base. Clonidine hydrochloride tablets USP contain the following inactive ingredients: lactose, magnesium stearate, microcrystalline cellulose, pregelatinized starch, and sodium starch glycolate. The 0.1 mg also contains D&C yellow #10 aluminum lake, and the 0.3 mg contains D&C yellow #10 aluminum lake and FD&C blue #1 aluminum lake. Clonidine hydrochloride is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-(2,6-dichlorophenylamino)-2-imidazoline hydrochloride. The following is the structural formula: C 9 H 9 Cl 2 N 3 • HCl Mol. Wt. 266.56 Clonidine hydrochloride is an odorless, bitter, white, crystalline substance soluble in water and alcohol. the structural formula for Clonidine hydrochloride is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-(2,6-dichlorophenylamino)-2-imidazoline hydrochloride.
How Supplied
HOW SUPPLIED Clonidine hydrochloride tablets USP are supplied as follows: Clonidine hydrochloride tablets USP 0.1 mg, yellow, round, debossed MP 657 on one side and plain on the other side. Bottles of 100 NDC 52817-180-10 Bottles of 500 NDC 52817-180-50 Bottles of 1000 NDC 52817-180-00 Clonidine hydrochloride tablets USP 0.2 mg, white, round, debossed MP 658 on one side and plain on the other side. Bottles of 100 NDC 52817-181-10 Bottles of 500 NDC 52817-181-50 Bottles of 1000 NDC 52817-181-00 Clonidine hydrochloride tablets USP 0.3 mg, green, round, debossed MP 659 on one side and plain on the other side. Bottles of 100 NDC 52817-182-10 Bottles of 500 NDC 52817-182-50 Bottles of 1000 NDC 52817-182-00 Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature] DISPENSE IN TIGHT, LIGHT-RESISTANT CONTAINER. Add ress medical inquiries to: 1-877-541-5504 . Manufactured by: Frontida BioPharm, Inc Philadelphia, PA 19124 Distributed by: TruPharma, LLC Tampa, FL 33609 Rev00, March 2017
Indications & Usage
INDICATIONS AND USAGE Clonidine hydrochloride tablets USP are indicated in the treatment of hypertension. Clonidine hydrochloride tablets USP may be employed alone or concomitantly with other antihypertensive agents.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adults The dose of clonidine hydrochloride tablets USP must be adjusted according to the patient’s individual blood pressure response. The following is a general guide to its administration. Initial Dose 0.1 mg tablet twice daily (morning and bedtime). Elderly patients may benefit from a lower initial dose. Maintenance Dose Further increments of 0.1 mg per day may be made at weekly intervals if necessary until the desired response is achieved. Taking the larger portion of the oral daily dose at bedtime may minimize transient adjustment effects of dry mouth and drowsiness. The therapeutic doses most commonly employed have ranged from 0.2 mg to 0.6 mg per day given in divided doses. Studies have indicated that 2.4 mg is the maximum effective daily dose, but doses as high as this have rarely been employed. Renal Impairment Patients with renal impairment may benefit from a lower initial dose. Patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
