Drug Catalog - Product Detail
CLONIDINE HCI TB 0.2MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00228-2128-50 | ACTAVIS PHARMA | 500 | 0.2MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
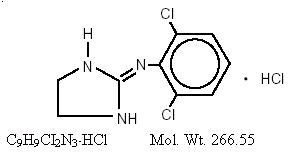
DESCRIPTION Clonidine hydrochloride, USP is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg, and 0.3 mg. The 0.1 mg tablet is equivalent to 0.087 mg of the free base. The following inactive ingredients are contained in these products: corn starch, D&C yellow #10 Aluminum Lake, FD&C yellow #6 Aluminum Lake (Sunset Yellow Lake), lactose monohydrate, magnesium stearate, and sodium starch glycolate. Clonidine hydrochloride, USP is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-(2,6-dichlorophenylamino)-2-imidazoline hydrochloride. The following is the structural formula: Clonidine hydrochloride, USP is an odorless, bitter, white, crystalline substance soluble in water and alcohol. Structure
How Supplied
HOW SUPPLIED Clonidine hydrochloride tablets, USP are supplied as follows: 0.1 mg — Each orange, round tablet imprinted with and 127 on one side and bisect on the other side contains 0.1 mg of clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2127-10) and 500 (NDC 0228-2127-50). 0.2 mg — Each orange, round tablet imprinted with on one side and 128 and bisect on the other side contains 0.2 mg of clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2128-10) and 500 (NDC 0228-2128-50). 0.3 mg — Each orange, round tablet imprinted with on one side and 129 and bisect on the other side contains 0.3 mg of clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2129-10). Dispense in a tight, light-resistant container as defined in the USP. Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). Manufactured by: Actavis Elizabeth LLC 200 Elmora Avenue Elizabeth, NJ 07207 USA 40-9183 Revised — July 2012 usp-2 usp-3 usp-4
Indications & Usage
INDICATIONS AND USAGE Clonidine hydrochloride tablets, USP are indicated in the treatment of hypertension. Clonidine hydrochloride tablets may be employed alone or concomitantly with other antihypertensive agents.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adults: The dose of clonidine hydrochloride tablets, USP must be adjusted according to the patient’s individual blood pressure response. The following is a general guide to its administration. Initial Dose: 0.1 mg tablet twice daily (morning and bedtime). Elderly patients may benefit from a lower initial dose. Maintenance Dose: Further increments of 0.1 mg per day may be made at weekly intervals if necessary until the desired response is achieved. Taking the larger portion of the oral daily dose at bedtime may minimize transient adjustment effects of dry mouth and drowsiness. The therapeutic doses most commonly employed have ranged from 0.2 mg to 0.6 mg per day given in divided doses. Studies have indicated that 2.4 mg is the maximum effective daily dose, but doses as high as this have rarely been employed. Renal Impairment: Patients with renal impairment may benefit from a lower initial dose. Patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis. For questions regarding this product call Actavis at 1-800-432-8534.
