Drug Catalog - Product Detail
CLOBETASOL PROP OINT USP 0.05% 15GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0530-17 | GLENMARK PHARMACEUTICALS | 15 | 0.05% | OINTMENT |
PACKAGE FILES


Generic Name
CLOBETASOL PROPIONATE
Substance Name
CLOBETASOL PROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA208933
Description
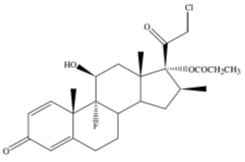
DESCRIPTION Clobetasol Propionate Ointment USP, 0.05% contains the active compound clobetasol propionate USP, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity. Chemically, clobetasol propionate USP is 21-Chloro-9-fluoro-11β,17-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-propionate, and it has the following structural formula: Clobetasol propionate USP has the empirical formula of C 25 H 32 ClFO 5 and a molecular weight of 466.97 g/mol. It is a white to almost white, crystalline powder, practically insoluble in water, slightly soluble in benzene and diethyl ether; sparingly soluble in ethanol; freely soluble in acetone, in dimethylsulfoxide, in chloroform, in methanol and in dioxane. Clobetasol Propionate Ointment USP, 0.05% contains clobetasol propionate USP 0.5 mg/g in a base of propylene glycol, sorbitan sesquioleate, and white petrolatum. image-01
How Supplied
HOW SUPPLIED Clobetasol Propionate Ointment USP, 0.05% is available as follows: NDC 68462-530-17 15 g tube (1 tube per carton) NDC 68462-530-35 30 g tube (1 tube per carton) NDC 68462-530-47 45 g tube (1 tube per carton) NDC 68462-530-65 60 g tube (1 tube per carton) Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not refrigerate and avoid freezing. Manufactured by: Glenmark Pharmaceuticals Limited Baddi, Himachal Pradesh 173205, India Manufactured for: Glenmark Pharmaceuticals Inc., USA Mahwah, NJ 07430 Questions? 1 (888) 721-7115 www.glenmarkpharma-us.com August 2020 Logo.jpg
Indications & Usage
INDICATIONS AND USAGE Clobetasol propionate ointment is a super-high potency corticosteroid formulation indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Treatment beyond 2 consecutive weeks is not recommended, and the total dosage should not exceed 50 grams per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Use in pediatric patients under 12 years of age is not recommended. As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply a thin layer of clobetasol propionate ointment to the affected skin areas twice daily and rub in gently and completely. (See INDICATIONS AND USAGE ). Clobetasol propionate ointment is a super-high potency topical corticosteroid; therefore, treatment should be limited to 2 consecutive weeks and amounts greater than 50 grams per week should not be used. As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Clobetasol propionate ointment should not be used with occlusive dressings. Geriatric Use: In studies where geriatric patients (65 years of age or older, see PRECAUTIONS ) have been treated with clobetasol propionate ointment, safety did not differ from that in younger patients; therefore, no dosage adjustment is recommended.
