Drug Catalog - Product Detail
CLOBETASOL PROP CREAM 0.05% 60GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0529-65 | GLENMARK PHARMACEUTICALS | 60 | 0.05% | CREAM |
PACKAGE FILES
Generic Name
CLOBETASOL PROPIONATE
Substance Name
CLOBETASOL PROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA209095
Description
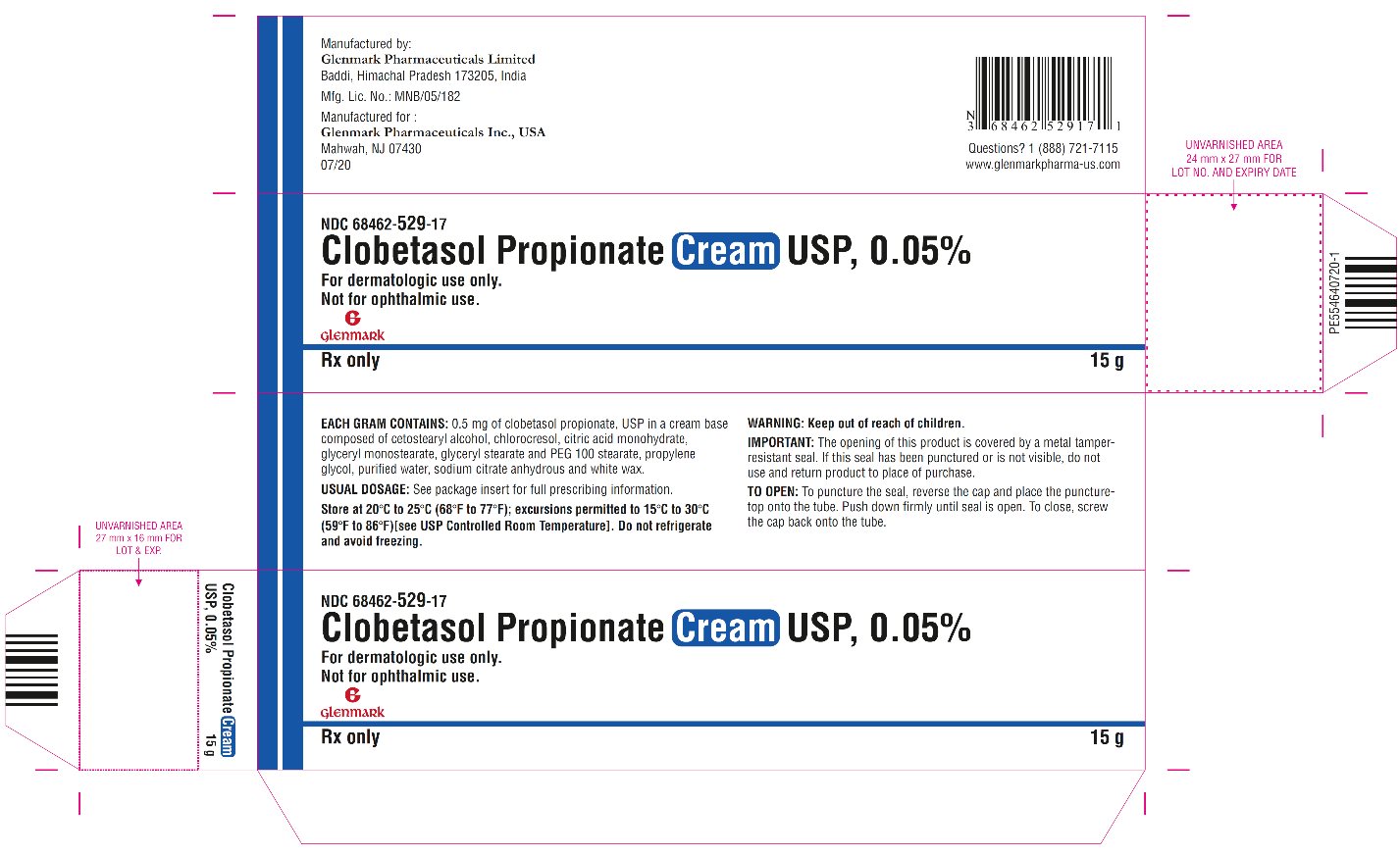
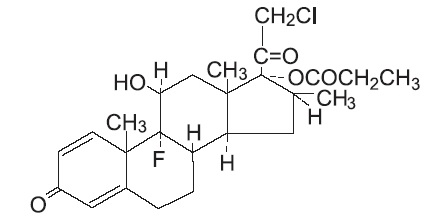
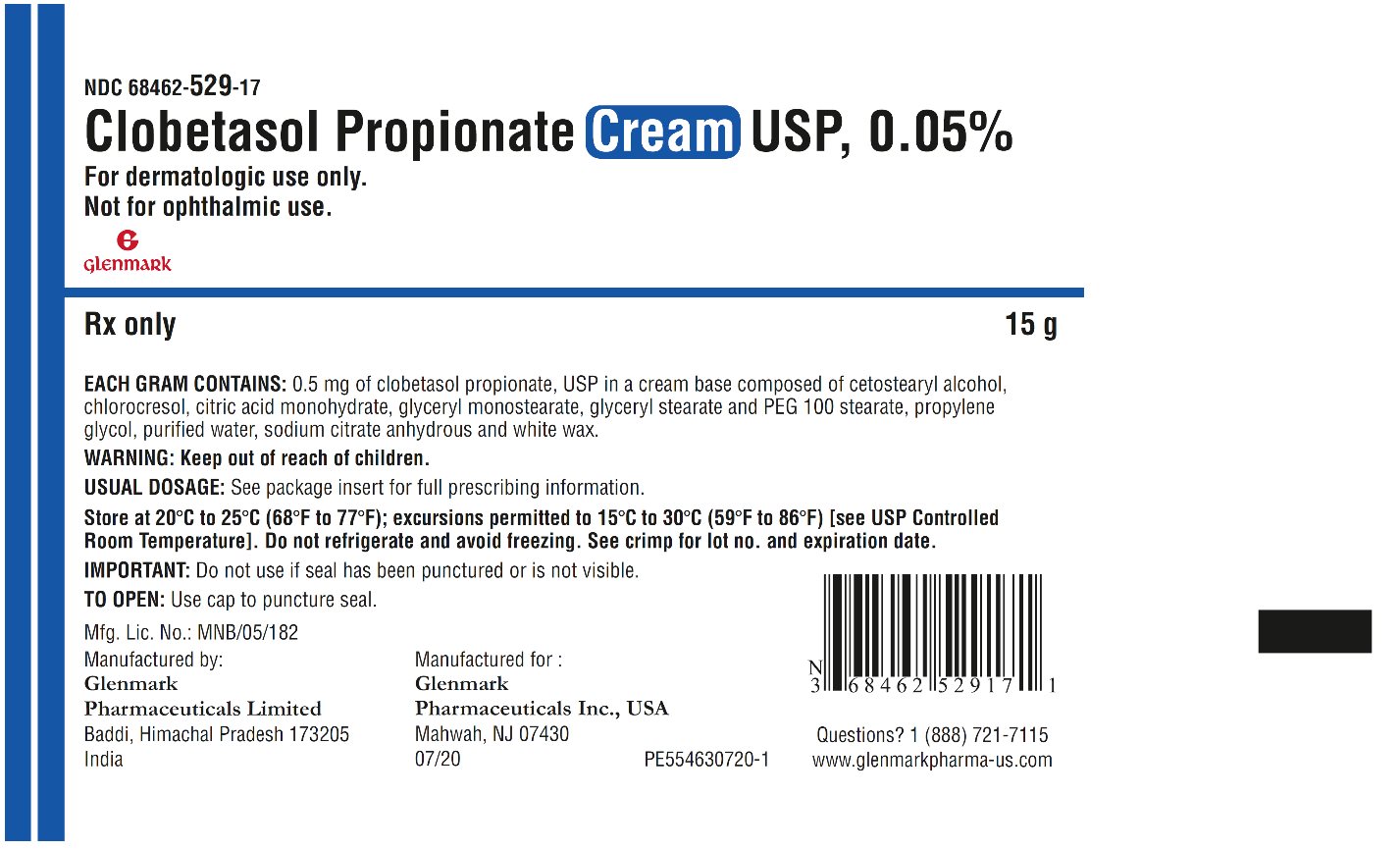
DESCRIPTION: Clobetasol Propionate Cream USP, 0.05% contains the active compound clobetasol propionate, USP, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity. Chemically, clobetasol propionate, USP is 21-chloro-9-fluoro- 11β,17-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-propionate, and it has the following structural formula: Clobetasol propionate, USP has the molecular formula C 25 H 32 CIFO 5 and a molecular weight of 466.97 g/mol. It is a white to almost white, crystalline powder, practically insoluble in water, slightly soluble in benzene and diethyl ether; sparingly soluble in ethanol; freely soluble in acetone, in dimethylsulfoxide, in chloroform, in methanol and in dioxane. Clobetasol Propionate Cream USP, 0.05% contains clobetasol propionate, USP 0.5 mg/g in a cream base composed of cetostearyl alcohol, chlorocresol, citric acid monohydrate, glyceryl monostearate, glyceryl stearate and PEG 100 stearate, propylene glycol, purified water, sodium citrate anhydrous and white wax. clobetasol-propionate-structure.jpg
How Supplied
HOW SUPPLIED: Clobetasol Propionate Cream USP, 0.05% is supplied in 15 g tubes (NDC 68462-529-17), 30 g tubes (NDC 68462-529-35), 45 g tubes (NDC 68462-529-47) and 60 g tubes (NDC 68462-529-65). Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not refrigerate and avoid freezing . Rx only Manufactured by: Glenmark Pharmaceuticals Limited Baddi, Himachal Pradesh 173205, India Manufactured for: Glenmark Pharmaceuticals Inc., USA Mahwah, NJ 07430 Questions? 1 (888) 721-7115 www.glenmarkpharma-us.com July 2020
Indications & Usage
INDICATIONS AND USAGE: Clobetasol propionate cream is a super-high potency corticosteroid formulations indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Treatment beyond 2 consecutive weeks is not recommended, and the total dosage should not exceed 50 g/week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Use in pediatric patients under 12 years of age is not recommended. As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
Dosage and Administration
DOSAGE AND ADMINISTRATION: Apply a thin layer of clobetasol propionate cream to the affected skin areas twice daily and rub in gently and completely (see INDICATIONS AND USAGE ). Clobetasol propionate cream are super-high potency topical corticosteroids; therefore, treatment should be limited to 2 consecutive weeks and amounts greater than 50 g/week should not be used. As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Clobetasol propionate cream should not be used with occlusive dressings. Geriatric Use: In studies where geriatric patients (65 years of age or older, see PRECAUTIONS ) have been treated with clobetasol propionate cream, safety did not differ from that in younger patients; therefore, no dosage adjustment is recommended.
