Drug Catalog - Product Detail
Clindamycin HCl Cap 300 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 63304-0693-01 | SUN PHARMACEUTICALS | 100 | 300MG | CAPSULE |
PACKAGE FILES


Generic Name
CLINDAMYCIN HYDROCHLORIDE
Substance Name
CLINDAMYCIN HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA065061
Description
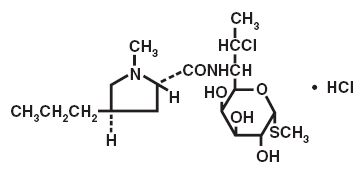
DESCRIPTION Clindamycin hydrochloride is the hydrated hydrochloride salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compound lincomycin. Clindamycin hydrochloride capsules, USP contain clindamycin hydrochloride, USP equivalent to 150 mg or 300 mg of clindamycin. Inactive ingredients: 150 mg - black iron oxide, corn starch, D&C Yellow #10, FD&C Blue no. 1, gelatin, lactose monohydrate, magnesium stearate, potassium hydroxide, propylene glycol, shellac, talc, and titanium dioxide; 300 mg - black iron oxide, corn starch, FD&C Blue no. 1, gelatin, lactose monohydrate, magnesium stearate, potassium hydroxide, propylene glycol, shellac, talc, and titanium dioxide. The structural formula is represented below: C 18 H 33 ClN 2 O 5 S•HCl M.W. 461.45 The chemical name for clindamycin hydrochloride is Methyl 7-chloro-6, 7, 8-trideoxy-6-(1-methyl- trans -4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L- threo -α-D- galacto -octopyranoside monohydrochloride. structure
How Supplied
HOW SUPPLIED Clindamycin hydrochloride capsules, USP are available in the following strengths, colors and sizes: Clindamycin hydrochloride capsules, USP, 150 mg are size ‘1’ capsules with turquoise blue opaque cap and light green body imprinted with “RX692” on cap and body in black ink containing white to off white powder. They are supplied as follows: NDC 63304-692-03 Bottles of 10 NDC 63304-692-01 Bottles of 100 NDC 63304-692-05 Bottles of 500 NDC 63304-692-77 Carton of 100 (10 X 10 Unit-Dose blister) Clindamycin hydrochloride capsules, USP, 300 mg are size ‘0’ capsules with turquoise blue opaque cap and turquoise blue opaque body imprinted with “RX693” on cap and body in black ink containing white to off white powder. They are supplied as follows: NDC 63304-693-03 Bottles of 10 NDC 63304-693-16 Bottles of 16 NDC 63304-693-01 Bottles of 100 NDC 63304-693-62 Carton of 60 (3 X 20 Unit-Dose blister) NDC 63304-693-77 Carton of 100 (10 X 10 Unit-Dose blister) NDC 63304-693-05 Bottles of 500 Store at 20° - 25° C (68° - 77° F) [See USP Controlled Room Temperature]. To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Indications & Usage
INDICATIONS AND USAGE Clindamycin hydrochloride capsules, USP are indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. Clindamycin hydrochloride capsules, USP are also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, as described in the BOXED WARNING , before selecting clindamycin, the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin). Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis, and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, nongonococcal tubo-ovarian abscess, pelvic cellulitis, and postsurgical vaginal cuff infection. Streptococci: Serious respiratory tract infections; serious skin and soft tissue infections. Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections. Pneumococci: Serious respiratory tract infections. Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin. To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride capsules, USP and other antibacterial drugs, clindamycin hydrochloride capsules, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION If significant diarrhea occurs during therapy, this antibacterial drug should be discontinued (See BOXED WARNING ). Administer clindamycin hydrochloride capsules with a full glass of water (6 to 8 ounces, approximately 200 to 250 mL) and at least 30 minutes before lying down to reduce the potential for esophageal irritation (See ADVERSE REACTIONS ). Adults: Serious infections —150 to 300 mg every 6 hours. More severe infections - 300 to 450 mg every 6 hours. Pediatric Patients (who are able to swallow capsules): Serious infections - 8 to 16 mg/kg/day (4 to 8 mg/lb/day) divided into three or four equal doses. More severe infections - 16 to 20 mg/kg/day (8 to 10 mg/lb/day) divided into three or four equal doses. Clindamycin should be dosed based on total body weight regardless of obesity. Clindamycin hydrochloride capsules are not suitable for pediatric patients who are unable to swallow them whole. The capsules do not provide exact mg/kg doses therefore it may be necessary to use the clindamycin palmitate oral solution in some cases. Serious infections due to anaerobic bacteria are usually treated with clindamycin injection. However, in clinically appropriate circumstances, the physician may elect to initiate treatment or continue treatment with clindamycin hydrochloride capsules. In cases of β-hemolytic streptococcal infections, treatment should continue for at least 10 days.
