Drug Catalog - Product Detail
CLARITHROMYCIN ER TABS. TB 500MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-2805-60 | ACTAVIS PHARMA | 60 | 500MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
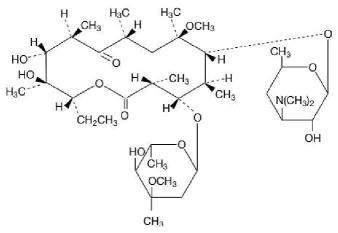
DESCRIPTION Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6- 0 -methylerythromycin. The molecular formula is C 38 H 69 NO 13 , and the molecular weight is 747.96. The structural formula is: Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water. Each yellow oval film-coated clarithromycin extended-release tablet, for oral administration, contains 500 mg of clarithromycin and the following inactive ingredients: compressible sugar, D&C yellow #10 Lake, glycerol monostearate, polyethylene glycol 3000, polyvinyl alcohol, sodium phosphate monobasic (anhydrous), talc and titanium dioxide. Meets USP requirements for dissolution test 2. Clarithromycin structural formula
How Supplied
HOW SUPPLIED Clarithromycin extended-release tablets, USP are supplied as yellow, film coated, oval shaped tablets debossed with and "777" on one side. Bottles of 60 (NDC 0591-2805-60), bottles of 500 (NDC 0591-2805-05), bottles of 1000 (NDC 0591-2805-10). Store clarithromycin extended-release tablets, USP at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Company Logo
Indications & Usage
INDICATIONS AND USAGE To reduce the development of drug-resistant bacteria and maintain the effectiveness of clarithromycin extended-release tablets, USP and other antibacterial drugs, clarithromycin extended-release tablets, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Adults (Clarithromycin extended-release tablets, USP) Clarithromycin extended-release tablets, USP are indicated for the treatment of adults with mild to moderate infection caused by susceptible strains of the designated microorganisms in the conditions listed below: Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis , or Streptococcus pneumoniae. Acute bacterial exacerbation of chronic bronchitis due to Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis , or Streptococcus pneumoniae. Community-Acquired Pneumonia due to Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, Streptococcus pneumoniae, Chlamydophila pneumoniae (TWAR), or Mycoplasma pneumoniae. THE EFFICACY AND SAFETY OF CLARITHROMYCIN EXTENDED-RELEASE TABLETS, USP IN TREATING OTHER INFECTIONS FOR WHICH OTHER FORMULATIONS OF CLARITHROMYCIN ARE APPROVED HAVE NOT BEEN ESTABLISHED.
Dosage and Administration
DOSAGE AND ADMINISTRATION Clarithromycin extended-release tablets should be taken with food. Clarithromycin extended-release tablets should be swallowed whole and not chewed, broken or crushed. Clarithromycin may be administered without dosage adjustment in the presence of hepatic impairment if there is normal renal function. In patients with severe renal impairment (CL CR < 30 mL/min), the dose of clarithromycin should be reduced by 50%. However, when patients with moderate or severe renal impairment are taking clarithromycin concomitantly with atazanavir or ritonavir, the dose of clarithromycin should be reduced by 50% or 75% for patients with CL CR of 30 to 60 mL/min or < 30 mL/min, respectively. ADULT DOSAGE GUIDELINES Clarithromycin Extended-release Tablets Infection Dosage (q24h) Duration (days) Acute maxillary sinusitis due to 2 x 500 mg 14 H. influenzae M. catarrhalis S. pneumoniae Acute exacerbation of chronic bronchitis due to: H. influenzae 2 x 500 mg 7 H. parainfluenzae 2 x 500 mg 7 M. catarrhalis 2 x 500 mg 7 S. pneumoniae 2 x 500 mg 7 Community-Acquired Pneumonia due to: H. influenzae 2 x 500 mg 7 H. parainfluenzae 2 x 500 mg 7 M. catarrhalis 2 x 500 mg 7 S. pneumoniae 2 x 500 mg 7 C. pneumoniae 2 x 500 mg 7 M. pneumoniae 2 x 500 mg 7
