Drug Catalog - Product Detail
CITALOPRAM HBR TB 40MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0824-12 | CIPLA USA | 500 | 40MG | TABLET |
PACKAGE FILES






Generic Name
CITALOPRAM
Substance Name
CITALOPRAM HYDROBROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077534
Description
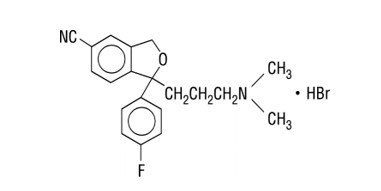
11 DESCRIPTION Citalopram tablet contains citalopram hydrobromide, an orally administered selective serotonin reuptake inhibitor (SSRI) with a chemical structure unrelated to that of other SSRIs or of tricyclic, tetracyclic, or other available antidepressant agents. Citalopram hydrobromide is a racemic bicyclic phthalane derivative designated ((±)-1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile, hydrobromide with the following structural formula: The molecular formula is C20H22BrFN2O and its molecular weight is 405.35. Citalopram hydrobromide occurs as a fine, white to off-white powder. Citalopram hydrobromide is sparingly soluble in water and soluble in ethanol. Citalopram is available only in tablet dosage form. Citalopram tablets, USP 10 mg are film-coated, round tablets containing citalopram hydrobromide in strengths equivalent to 10 mg citalopram base. Citalopram hydrobromide, USP 20 mg and 40 mg tablets are film-coated, round, scored tablets containing citalopram hydrobromide in strengths equivalent to 20 mg or 40 mg citalopram base. Their strengths reflect their citalopram base equivalent content. The 10 mg, 20 mg and 40 mg strength tablets contain 12.49 mg, 24.98 mg and 49.96 mg of citalopram hydrobromide, respectively. The tablets also contain the following. Inactive ingredients : copovidone, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, opadry beige (HPMC 2910/hypromellose 6cp, titanium dioxide, macrogol/Peg400, iron oxide yellow and iron oxide red), opadry pink (HPMC 2910/hypromellose 6cP, titanium dioxide, macrogol/Peg400 and iron oxide red) and opadry white (titanium dioxide, HPMC 2910/hypromellose 3cp, HPMC 2910/ hypromellose 6cp, Macrogol/Peg400 and Polysorbate 80) are used as coloring agents in the beige (10 mg) and pink (20 mg) and white (40 mg) tablets. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Citalopram Tablets, USP 10 mg: They are supplied in Bottle of 30 NDC # 69097-822-02, Bottle of 100 NDC # 69097-822-07, Bottle of 500 NDC # 69097-822-12 and Bottle of 1,000 NDC # 69097-822-15 Beige, film coated round, bi-convex tablets debossed with IG on one side and “206” on the other. 20 mg:They are supplied in Bottle of 30 NDC # 69097-823-02, Bottle of 100 NDC # 69097-823-07, Bottle of 500 NDC # 69097-823-12 and Bottle of 1,000 NDC # 69097-823-15 Pink, film coated, round, bi-convex tablets debossed with I on the left side of bisect and G on right side of bisect on one side and “207” on the other. 40 mg: They are supplied in Bottle of 30 NDC # 69097-824-02, Bottle of 100 NDC # 69097-824-07, Bottle of 500 NDC # 69097- 824-12 and Bottle of 1,000 NDC # 69097-824-15 White, film coated, round, bi-convex tablets debossed with I on the left side of bisect and G on right side of bisect on one side and “208” on the other Storage and Handling Citalopram tablets should be stored at 20-25°C (68 to 77°F); excursions permitted between 15 and 30°C (59-86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Citalopram is indicated for the treatment of major depressive disorder (MDD) in adults [see Clinical Studies ( 14 )] . Citalopram is a selective serotonin reuptake inhibitor (SSRI) indicated for the treatment of major depressive disorder (MDD) in adults ( 1 ).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Administer once daily with or without food ( 2 ). Initial dosage is 20 mg once daily; after one week may increase to maximum dosage of 40 mg once daily ( 2.1 ). Patients greater than 60 years of age, patients with hepatic impairment, and CYP2C19 poor metabolizers: maximum recommended dosage is 20 mg once daily ( 2.2 ). When discontinuing Citalopram, reduce dosage gradually ( 2.4 , 5.6 ) . 2.1 Recommended Dosage Administer Citalopram once daily, with or without food, at an initial dosage of 20 mg once daily, with an increase to a maximum dosage of 40 mg once daily at an interval of no less than one week. Dosages above 40 mg once daily are not recommended due to the risk of QT prolongation [see Warnings and Precautions ( 5.2 )] . 2.2 Screen for Bipolar Disorder Prior to Starting Citalopram Prior to initiating treatment with Citalopram or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [See Warnings and Precautions ( 5.5 )] . 2.3 Recommended Dosage for Specific Populations The maximum recommended dosage of Citalopram for patients who are greater than 60 years of age, patients with hepatic impairment, and for CYP2C19 poor metabolizers is 20 mg once daily [see Warnings and Precautions ( 5.2 ), Clinical Pharmacology ( 12.3 )] . 2.4 Dosage Modifications with Concomitant Use of CYP2C19 Inhibitors The Maximum recommended dosage of Citalopram when used concomitantly with a CYP2C19 inhibitor is 20 mg once daily [see Warnings and Precautions ( 5.2 ), Drug Interactions ( 7 ) ]. 2.5 Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of therapy with Citalopram. Conversely, at least 14 days must elapse after stopping Citalopram before starting an MAOI antidepressant [see Contraindications ( 4 ) and Warnings and Precautions ( 5.3 )]. 2.6 Discontinuing Treatment with Citalopram Adverse reactions may occur upon discontinuation of Citalopram [see Warnings and Precautions ( 5.6 )]. Gradually reduce the dosage rather than stopping Citalopram abruptly whenever possible.
