Drug Catalog - Product Detail
CIPROFLOXACIN 500MG TABLETS 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16252-0515-01 | ACTAVIS PHARMA | 100 | 500MG | TABLET |
PACKAGE FILES





Generic Name
CIPROFLOXACIN
Substance Name
CIPROFLOXACIN HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA076794
Description
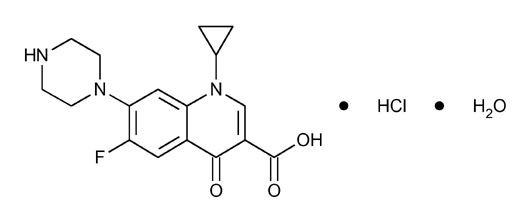
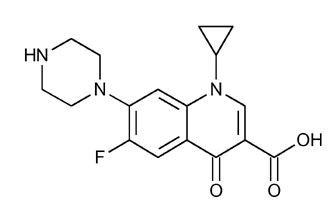
11 DESCRIPTION Ciprofloxacin tablets, USP are synthetic antimicrobial agents for oral administration. Ciprofloxacin hydrochloride, USP, a fluoroquinolone, is the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3quinolinecarboxylic acid. It is a faintly yellowish to light yellow crystalline substance with a molecular weight of 385.82. Its molecular formula is C 17 H 18 FN 3 O 3 •HCl•H 2 O and its chemical structure is as follows: Ciprofloxacin, USP is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its molecular formula is C 17 H 18 FN 3 O 3 and its molecular weight is 331.34. It is a faintly yellowish to light yellow crystalline substance and its chemical structure is as follows: Ciprofloxacin film-coated tablets are available in 250 mg, 500 mg and 750 mg (ciprofloxacin equivalent) strengths. Ciprofloxacin tablets, USP are white to off-white. Each ciprofloxacin film-coated tablet contains 250 mg (equivalent to 291 mg ciprofloxacin hydrochloride monohydrate), 500 mg of ciprofloxacin (equivalent to 582 mg ciprofloxacin hydrochloride monohydrate), and 750 mg of ciprofloxacin (equivalent to 873 mg ciprofloxacin hydrochloride monohydrate). The inactive ingredients are corn starch, crospovidone, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, pregelatinized starch (maize), purified water, silicon dioxide, talc, and titanium dioxide. 1 1
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Ciprofloxacin tablets USP, 250 mg are available as white to off-white, round, biconvex film-coated, unscored tablets, with “ ”on one side and on the other side containing 250 mg ciprofloxacin. Ciprofloxacin tablets USP, 500 mg are available as white to off-white, capsule shaped, biconvex film-coated, unscored tablets, with “ ”on one side and “CR 500” on the other side containing 500 mg ciprofloxacin. Ciprofloxacin tablets USP, 750 mg are available as white to off-white, capsule shaped, biconvex film-coated, unscored tablets, with “ ”on one side and “CR 750” on the other side containing 750 mg ciprofloxacin. Ciprofloxacin tablets USP, 250 mg, 500 mg, and 750 mg are available in the following pack types. Strength NDC Code Bottles of 100 250 mg NDC 16252-514-01 500 mg NDC 16252-515-01 Bottles of 50 750 mg NDC 16252-516-05 Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature]. 1 250 1 1
Indications & Usage
1 INDICATIONS AND USAGE Ciprofloxacin tablets are a fluoroquinolone antibacterial indicated in adults (18 years of age and older) with the following infections caused by designated, susceptible bacteria and in pediatric patients where indicated: Skin and Skin Structure Infections ( 1.1 ) Bone and Joint Infections ( 1.2 ) Complicated Intra-Abdominal Infections ( 1.3 ) Infectious Diarrhea ( 1.4 ) Typhoid Fever (Enteric Fever) ( 1.5 ) Uncomplicated Cervical and Urethral Gonorrhea ( 1.6 ) Inhalational Anthrax post-exposure in adult and pediatric patients ( 1.7 ) Plague in adult and pediatric patients ( 1.8 ) Chronic Bacterial Prostatitis ( 1.9 ) Lower Respiratory Tract Infections ( 1.10 ) ○ Acute Exacerbation of Chronic Bronchitis Urinary Tract Infections ( 1.11 ) ○ Urinary Tract Infections (UTI) ○ Acute Uncomplicated Cystitis ○ Complicated UTI and Pyelonephritis in Pediatric Patients Acute Sinusitis ( 1.12 ) Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of ciprofloxacin tablets and other antibacterial drugs, ciprofloxacin tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. ( 1.13 ) 1.1 Skin and Skin Structure Infections Ciprofloxacin tablets are indicated in adult patients for treatment of skin and skin structure infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Proteus vulgaris, Providencia stuartii, Morganella morganii, Citrobacter freundii, Pseudomonas aeruginosa, methicillinsusceptible Staphylococcus aureus, methicillin-susceptible Staphylococcus epidermidis, or Streptococcus pyogenes. 1.2 Bone and Joint Infections Ciprofloxacin tablets are indicated in adult patients for treatment of bone and joint infections caused by Enterobacter cloacae, Serratia marcescens, or Pseudomonas aeruginosa. 1.3 Complicated Intra-Abdominal Infections Ciprofloxacin tablets are indicated in adult patients for treatment of complicated intra-abdominal infections (used in combination with metronidazole) caused by Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, or Bacteroides fragilis. 1.4 Infectious Diarrhea Ciprofloxacin tablets are indicated in adult patients for treatment of infectious diarrhea caused by Escherichia coli (enterotoxigenic isolates), Campylobacter jejuni, Shigella boydii † , Shigella dysenteriae, Shigella flexneri or Shigella sonnei † when antibacterial therapy is indicated. † Although treatment of infections due to this organism in this organ system demonstrated a clinically significant outcome, efficacy was studied in fewer than 10 patients. 1.5 Typhoid Fever (Enteric Fever) Ciprofloxacin tablets are indicated in adult patients for treatment of typhoid fever (enteric fever ) caused by Salmonella typhi. The efficacy of ciprofloxacin in the eradication of the chronic typhoid carrier state has not been demonstrated. 1.6 Uncomplicated Cervical and Urethral Gonorrhea Ciprofloxacin tablets are indicated in adult patients for treatment of uncomplicated cervical and urethral gonorrhea due to Neisseria gonorrhoeae [see Warnings and Precautions (5.17) ]. 1.7 Inhalational Anthrax (Post-Exposure) Ciprofloxacin tablets are indicated in adults and pediatric patients from birth to 17 years of age for inhalational anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis. Ciprofloxacin serum concentrations achieved in humans served as a surrogate endpoint reasonably likely to predict clinical benefit and provided the initial basis for approval of this indication. 1 Supportive clinical information for ciprofloxacin for anthrax post-exposure prophylaxis was obtained during the anthrax bioterror attacks of October 2001 [ s ee Clinical Studies (14.2) ] . 1.8 Plague Ciprofloxacin tablets are indicated for treatment of plague, including pneumonic and septicemic plague, due to Yersinia pestis (Y. pestis) and prophylaxis for plague in adults and pediatric patients from birth to 17 years of age. Efficacy studies of ciprofloxacin could not be conducted in humans with plague for feasibility reasons. Therefore this indication is based on an efficacy study conducted in animals only [see Clinical Studies (14.3) ] . 1.9 Chronic Bacterial Prostatitis Ciprofloxacin tablets are indicated in adult patients for treatment of chronic bacterial prostatitis caused by Esc h er i ch i a co l i or P ro t eus m i ra b i l i s. 1.10 Lower Respiratory Tract Infections Ciprofloxacin tablets are indicated in adult patients for treatment of lower respiratory tract infections caused by Esche r i c h i a co l i , Kl eb s i e l l a pneu m o n i ae, E n t e roba c t er c l oa c ae, P r o t e us mi ra b i l i s, Pseudo m on a s aeru g i nos a , H ae m op h il u s i n fl u enz a e, H ae m op h i l us p a ra i n fl u enz a e, or S t re p t oc oc cus p n eu m on i a e. Ciprofloxacin tablets are not a drug of first choice in the treatment of presumed or confirmed pneumonia secondary to S t re p t o c occ u s pn e u m on i a e. Ciprofloxacin tablets are indicated for the treatment of acute exacerbations of chronic bronchitis (AECB) caused by M or a xe l l a ca t a rrh a l i s. Because fluoroquinolones, including ciprofloxacin tablets, have been associated with serious adverse reactions [see W arn i ngs and P r eca u t i ons ( 5.1 to 5.16 ) ] and for some patients AECB is self-limiting, reserve ciprofloxacin tablets for treatment of AECB in patients who have no alternative treatment options . 1.11 Urinary Tract Infections U r i na r y Tr a ct I n f ec t i o n s i n Adu l t s Ciprofloxacin tablets are indicated in adult patients for treatment of urinary tract infections caused by Esche r i c h i a c o l i , K l eb s i e ll a p neu m o n i ae , E n t eroba c t e r c l oac a e , S e rr a ti a m arce s cen s , Pr o t eus m i r a b i l i s , P ro v i de n c i a re t t ge r i , M or g an e ll a m o rg a n ii , C i t roba c t e r ko s e r i , Ci t r obac t er f re u nd i i , P s eudo m o nas a e rug i nosa , methicillin-susceptible S t a p hy l o c occ u s e p i d e r m i d i s , S t aphy l o co c cus s apro p hy t i c u s , or E n t e roco c cus f aec a l i s . Acu t e U n co m p l i c a t ed C ys ti ti s Ciprofloxacin tablets are indicated in adult female patients for treatment of acute uncomplicated cystitis caused by Esche r i c h i a co l i or S t a ph y l ococc u s sa p rop h y t i cu s . Because fluoroquinolones, including ciprofloxacin tablets, have been associated with serious adverse reactions [ see Wa r n i n g s and P r e ca u ti o n s ( 5.1 to 5.16 ) ] and for some patients acute uncomplicated cystitis is self-limiting, reserve ciprofloxacin tablets for treatment of acute uncomplicated cystitis in patients who have no alternative treatment options. C o m p li c a t e d U r i n ary T ra c t I n f ec t i on a nd P y e l o n eph r i t i s i n Pe d i a t r i c Pa t i e n t s Ciprofloxacin tablets are indicated in pediatric patients aged one to 17 years of age for treatment of complicated urinary tract infections (cUTI) and pyelonephritis due to E s ch e r i c h i a c o l i [ s ee U s e i n Spe c i f i c Pop u l a ti o ns (8.4) ] . Although effective in clinical trials, ciprofloxacin is not a drug of first choice in the pediatric population due to an increased incidence of adverse reactions compared to controls, including reactions related to joints and/or surrounding tissues . Ciprofloxacin, like other fluoroquinolones, is associated with arthropathy and histopathological changes in weight-bearing joints of juvenile animals [see W arn i ngs and P rec a u t i ons (5.13) , Adverse Rea c t i ons (6.1) , U se i n S pe c i f i c P o pu l a ti o ns (8.4) and N on c l i n i c a l To x i c o l ogy (13.2) ] . 1.12 Acute Sinusitis Ciprofloxacin tablets are indicated in adult patients for treatment of acute sinusitis caused by H a e m oph i l us i n f l ue n zae, S t re p t o c occ u s pn e u m on i a e, or M ora x e l l a c a t a rrh a l i s. Because fluoroquinolones, including ciprofloxacin tablets, have been associated with serious adverse reactions [see Warnings and Precautions ( 5.1 to 5.16 )] and for some patients acute sinusitis is self-limiting, reserve ciprofloxacin tablets for treatment of acute sinusitis in patients who have no alternative treatment options. 1.13 Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of ciprofloxacin tablets and other antibacterial drugs, ciprofloxacin tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. If anaerobic organisms are suspected of contributing to the infection, appropriate therapy should be administered. Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing infection and to determine their susceptibility to ciprofloxacin. Therapy with ciprofloxacin tablets may be initiated before results of these tests are known; once results become available appropriate therapy should be continued. As with other drugs, some isolates of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with ciprofloxacin. Culture and susceptibility testing performed periodically during therapy will provide information not only on the therapeutic effect of the antimicrobial agent but also on the possible emergence of bacterial resistance.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Ciprofloxacin tablets should be administered orally as described in the appropriate Dosage Guidelines tables. Adu l t D o s a g e G u i d e l i n es I n f e c ti o n D o se F re qu e n cy Du ra ti o n Skin and Skin Structure 500 mg to 750 mg every 12 hours 7 to 14 days Bone and Joint 500 mg to 750 mg every 12 hours 4 to 8 weeks Complicated Intra-Abdominal 500 mg every 12 hours 7 to 14 days Infectious Diarrhea 500 mg every 12 hours 5 to 7 days Typhoid Fever 500 mg every 12 hours 10 days Uncomplicated Gonorrhea 250 mg single dose single dose Inhalational anthrax (postexposure) 500 mg every 12 hours 60 days Plague 500 mg to 750 mg every 12 hours 14 days Chronic Bacterial Prostatitis 500 mg every 12 hours 28 days Lower Respiratory Tract 500 mg to 750 mg every 12 hours 7 to 14 days Urinary Tract 250 mg to 500 mg every 12 hours 7 to 14 days Acute Uncomplicated Cystitis 250 mg every 12 hours 3 days Acute Sinusitis 500 mg every 12 hours 10 days Adults with creatinine clearance 30 mL/min to 50 mL/min 250 mg to 500 mg q 12 h ( 2.3 ) Adults with creatinine clearance 5 mL/min to 29 mL/min 250 mg to 500 mg q 18 h ( 2.3 ) Patients on hemodialysis or peritoneal dialysis 250 mg to 500 mg q 24 h (after dialysis) ( 2.3 ) Pediatric Oral Dosage Guidelines Infection Dose Frequency Duration Complicated UTI 10 mg/kg to 20 mg/kg and Pyelonephritis (maximum 750 mg Every 12 10 to 21 days (1 to 17 years of age) per dose) hours 15 mg/kg Inhalational Anthrax (maximum Every 12 60 days (Post-Exposure) 500 mg per dose) hours 15 mg/kg Plague (maximum 500 mg Every 8 to 14 days per dose) 12 hours 2.1 Dosage in Adults The determination of dosage and duration for any particular patient must take into consideration the severity and nature of the infection, the susceptibility of the causative microorganism, the integrity of the patient’s host-defense mechanisms, and the status of renal and hepatic function. Ciprofloxacin tablets may be administered to adult patients when clinically indicated at the discretion of the physician. Table 1: Adult Dosage Guidelines Infection Dose Frequency Usual Durations 1 Skin and Skin Structure 500 mg to 750 mg every 12 hours 7 to 14 days Bone and Joint 500 mg to 750 mg every 12 hours 4 to 8 weeks Complicated Intra-Abdominal 2 500 mg every 12 hours 7 to 14 days Infectious Diarrhea 500 mg every 12 hours 5 to 7 days Typhoid Fever 500 mg every 12 hours 10 days Uncomplicated Urethral and Cervical Gonococcal Infections 250 mg single dose single dose Inhalational anthrax (postexposure) 3 500 mg every 12 hours 60 days Plague 3 500 mg to 750 mg every 12 hours 14 days Chronic Bacterial Prostatitis 500 mg every 12 hours 28 days Lower Respiratory Tract Infections 500 mg to 750 mg every 12 hours 7 to 14 days Urinary Tract Infections 250 mg to 500 mg every 12 hours 7 to 14 days Acute Uncomplicated Cystitis 250 mg every 12 hours 3 days Acute Sinusitis 500 mg every 12 hours 10 days 1. Generally ciprofloxacin should be continued for at least 2 days after the signs and symptoms of infection have disappeared, except for inhalational anthrax (post-exposure). 2. Used in conjunction with metronidazole. 3. Begin drug administration as soon as possible after suspected or confirmed exposure. Conversion of Intravenous to Oral Dosing in Adults Patients whose therapy is started with ciprofloxacin intravenous may be switched to ciprofloxacin tablets when clinically indicated at the discretion of the physician (Table 2) [see Clinical Pharmacology ( 12.3 )]. Table 2: Equivalent AUC Dosing Regimens Ciprofloxacin Tablets Oral Dosage Equivalent Ciprofloxacin Intravenous Dosage 250 mg Tablet every 12 hours 200 mg intravenous every 12 hours 500 mg Tablet every 12 hours 400 mg intravenous every 12 hours 750 mg Tablet every 12 hours 400 mg intravenous every 8 hours 2.2 Dosage in Pediatric Patients Dosing and initial route of therapy (that is, intravenous or oral) for cUTI or pyelonephritis should be determined by the severity of the infection. Ciprofloxacin tablets should be administered as described in Table 3. Table 3: Pediatric Dosage Guidelines Infection Dose Frequency Total Duration 10 mg/kg to 20 mg/kg Complicated Urinary Tract or (maximum 750 mg per dose; not to be Pyelonephritis exceeded even in patients weighing more Every 12 hours 10 to 21 days 1 (patients from 1 to 17 years of age) than 51 kg) Inhalational Anthrax 15 mg/kg (Post-Exposure) 2 (maximum 500 mg per dose) Every 12 hours 60 days Plague 2,3 15 mg/kg Every 8 to 12 (maximum 500 mg per dose) hours 14 days 1. The total duration of therapy for cUTI and pyelonephritis in the clinical trial was determined by the physician. The mean duration of treatment was 11 days (range 10 to 21 days). 2. Begin drug administration as soon as possible after suspected or confirmed exposure. 3. Begin drug administration as soon as possible after suspected or confirmed exposure to Y. pestis . 2.3 Dosage Modifications in Patients with Renal Impairment Ciprofloxacin is eliminated primarily by renal excretion; however, the drug is also metabolized and partially cleared through the biliary system of the liver and through the intestine. These alternative pathways of drug elimination appear to compensate for the reduced renal excretion in patients with renal impairment. Nonetheless, some modification of dosage is recommended, particularly for patients with severe renal dysfunction. Dosage guidelines for use in patients with renal impairment are shown in Table 4. Table 4: Recommended Starting and Maintenance Doses for Adult Patients with Impaired Renal Function Creatinine Clearance (mL/min) Dose > 50 See Usual Dosage. 30 to 50 250 mg to 500 mg every 12 hours 5 to 29 250 mg to 500 mg every 18 hours Patients on hemodialysis or Peritoneal dialysis 250 mg to 500 mg every 24 hours (after dialysis) When only the serum creatinine concentration is known, the following formulas may be used to estimate creatinine clearance: Men - Creatinine clearance (mL/min) = Weight (kg) x (140–age) 72 x serum creatinine (mg/dL) Women - 0.85 x the value calculated for men. The serum creatinine should represent a steady state of renal function. In patients with severe infections and severe renal impairment, a unit dose of 750 mg may be administered at the intervals noted above. Patients should be carefully monitored. Pediatric patients with moderate to severe renal insufficiency were excluded from the clinical trial of cUTI and pyelonephritis. No information is available on dosing adjustments necessary for pediatric patients with moderate to severe renal insufficiency (that is, creatinine clearance of <50 mL/min/1.73m 2 ). 2.4 Important Administration Instructions With Multivalent Cations Administer ciprofloxacin tablets at least 2 hours before or 6 hours after magnesium/aluminum antacids; polymeric phosphate binders (for example, sevelamer, lanthanum carbonate) or sucralfate; Videx ® (didanosine) chewable/buffered tablets or pediatric powder for oral solution; other highly buffered drugs; or other products containing calcium, iron or zinc. With Dairy Products Concomitant administration of ciprofloxacin tablets with dairy products (like milk or yogurt) or calcium-fortified juices alone should be avoided since decreased absorption is possible; however, ciprofloxacin tablets may be taken with a meal that contains these products. Hydration of Patients Receiving Ciprofloxacin Tablets Assure adequate hydration of patients receiving ciprofloxacin tablets to prevent the formation of highly concentrated urine. Crystalluria has been reported with quinolones. Instruct the patient of the appropriate ciprofloxacin tablets administration [see Patient Counseling Information ( 17 )]. Missed Doses If a dose is missed, it should be taken anytime but not later than 6 hours prior to the next scheduled dose. If less than 6 hours remain before the next dose, the missed dose should not be taken and treatment should be continued as prescribed with the next scheduled dose. Double doses should not be taken to compensate for a missed dose.
