Drug Catalog - Product Detail
CHLORZOXAZONE TB 500MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-2520-01 | ACTAVIS PHARMA | 100 | 500MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
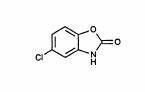
DESCRIPTION SECTION Chlorzoxazone USP is a centrally acting skeletal muscle relaxant, available as tablets of 500 mg for oral administration. Its chemical name is 5-Chloro-2-benzoxazolinone, and its structural formula is: C7H4CINO2 MW 169.57 Chlorzoxazone USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia. Chlorzoxazone tablets contain the inactive ingredients Docusate Sodium, Lactose (hydrous), Magnesium Stearate, Microcrystalline Cellulose, Pregelatinized Starch, Sodium Benzoate, and Sodium Starch Glycolate. image description
How Supplied
HOW SUPPLIED SECTION Chlorzoxazone tablets, USP are available as oblong, scored, white tablets debossed with WPI on one side and "39"-"68" on the other side and are packaged in bottles of 100 and 500. Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense contents with a child-resistant closure (as required) and in a tight container as defined in the USP/NF. Keep out of the reach of children. Manufactured By: Watson Pharma Private Limited Verna, Salcette Goa 403 722 INDIA Distributed By: Watson Pharma, Inc. Corona, CA 92880 USA Revised: November 2010 195609 PRINCIPAL DISPLAY PANEL NDC 0591-2520-01 Chlorzoxazone Tablets, USP 500 mg New NDC Watson 100 Tablets Rx only Each tablet contains: Chlorzoxazone USP, 500 mg Usual Dosage: See package insert for full prescribing information. Store at 20ºC-25ºC (68º-77ºF). [See USP Controlled Room Temperature.] KEEP TIGHTLY CLOSED. This is a bulk backage. Dispense contents with a child-resistant closure (as required) and in a tight container as defined in the USP/NF. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Manufactured By: Watson Pharma Private Limited Verna, Salcette Goa 403 722 INDIA Code No. GO/DRUGS/741 195607-1 Distributed By: Watson Pharma, Inc.
Indications & Usage
INDICATIONS & USAGE SECTION Chlorzoxazone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Chlorzoxazone does not directly relax tense skeletal muscles in man.
Dosage and Administration
DOSAGE & ADMINISTRATION SECTION Usual Adult Dosage One tablet three or four times daily. If adequate response is not obtained with this dose, it may be increased to one and one-half tablets (750 mg) three or four times daily. As improvement occurs dosage can usually be reduced.
