Drug Catalog - Product Detail
CHLORHEXIDINE GLUCONATE SOLUTION (DYNA-HEX 4) SOL 0.04 8OZ
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00116-1061-08 | XTTRIUM LABS (CS) | NA | NA | NA |
PACKAGE FILES

Generic Name
MUPIROCIN OINTMENT
Substance Name
Product Type
HUMAN PRESCRIPTION DRUG
Route
Application Number
Description
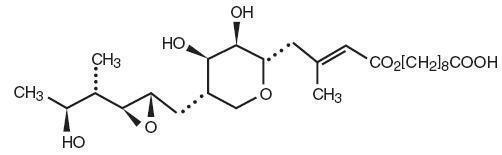
DESCRIPTION Each gram of Mupirocin Ointment USP, 2% contains 20 mg mupirocin in a bland water miscible ointment base (polyethylene glycol ointment, NF) consisting of polyethylene glycol 400 and polyethylene glycol 3350. Mupirocin is a naturally occurring antibiotic. The chemical name is ( E )-(2 S ,3 R ,4 R ,5 S )-5-[(2 S ,3 S ,4 S ,5 S )-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The molecular formula of mupirocin is C 26 H 44 O 9 and the molecular weight is 500.62. The chemical structure is: image description
How Supplied
HOW SUPPLIED Mupirocin Ointment USP, 2% is available as follows: 22 gram tube (NDC 45802-112-22) Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. *Bactroban Nasal ® is a registered trademark of GlaxoSmithKline.
Indications & Usage
INDICATIONS AND USAGE Mupirocin Ointment USP, 2% is indicated for the topical treatment of impetigo due to: S. aureus and S. pyogenes .
Dosage and Administration
DOSAGE AND ADMINISTRATION A small amount of Mupirocin Ointment USP, 2% should be applied to the affected area 3 times daily. The area treated may be covered with a gauze dressing if desired. Patients not showing a clinical response within 3 to 5 days should be re-evaluated.
