Drug Catalog - Product Detail
CHLORDIAZEPOXIDE W/CLIDINIUM CP 5/2.5MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 42582-0301-10 | BI COASTAL PHARMACEUTICAL | 100 | 5-2.5MG | NA |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
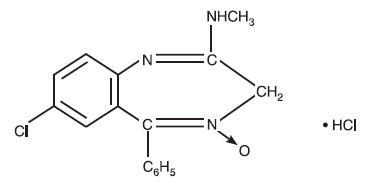
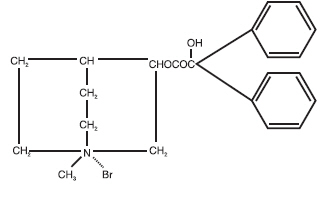
DESCRIPTION Chlordiazepoxide HCl and Clidinium Bromide combines in a single capsule formulation the antianxiety action of Chlordiazepoxide Hydrochloride and the anticholinergic/spasmolytic effects of Clidinium Bromide. Each Chlordiazepoxide HCl and Clidinium Bromide Capsule for oral administration contains 5 mg Chlordiazepoxide Hydrochloride and 2.5 mg Clidinium Bromide. Each capsule also contains D&C Yellow #10, FD&C Green #3, gelatin, lactose, starch, talc, and titanium dioxide. Chlordiazepoxide Hydrochloride is a versatile, therapeutic agent of proven value for the relief of anxiety and tension. It is indicated when anxiety, tension or apprehension are significant components of the clinical profile. It is among the safer of the effective psychopharmacologic compounds. Chlordiazepoxide Hydrochloride is 7-chloro-2-methylamino-5-phenyl- 3H-1, 4-benzodiazepine 4-oxide hydrochloride. A colorless, crystalline substance, it is soluble in water. It is unstable in solution and the powder must be protected from light. The molecular weight is 336.22. The structural formula of Chlordiazepoxide Hydrochloride is as follows: Clidinium Bromide is 3-hydroxy-1-methylquinuclidinium bromide benzilate, a synthetic anticholinergic agent which has been shown in experimental and clinical studies to have a pronounced antispasmodic and antisecretory effect on the gastrointestinal tract. Structurally Clidinium Bromide is: Chemical Structure Chlordiazepoxide Chemical Structure Clidinium
How Supplied
HOW SUPPLIED Chlordiazepoxide HCl and Clidinium Bromide Capsules are available in white opaque capsules, imprinted with "200", each containing 5 mg Chlordiazepoxide Hydrochloride and 2.5 mg Clidinium Bromide. Bottle of 100 NDC 42582-301-10 Bottle of 250 NDC 42582-301-16 Bottle of 1000 NDC 42582-301-20 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP/NF. Keep this and all medication out of the reach of children.
Indications & Usage
INDICATIONS AND USAGE Based on a review of this drug by the National Academy of Sciences – National Research Council and/or other information, FDA has classified the indications as follows: "Possibly" effective: as adjunctive therapy in the treatment of peptic ulcer and in the treatment of the irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis. Final classification of the less-than-effective indications requires further investigation.
Dosage and Administration
DOSAGE AND ADMINISTRATION Because of the varied individual responses to tranquilizers and anticholinergics, the optimum dosage of Chlordiazepoxide HCl and Clidinium Bromide Capsules varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects. The usual maintenance dose is 1 or 2 capsules orally, 3 or 4 times a day administered before meals and at bedtime. Geriatric Dosing Dosage should be limited to the smallest effective amount to preclude the development of ataxia, oversedation or confusion. The initial dose should not exceed 2 Chlordiazepoxide HCl and Clidinium Bromide Capsules per day, to be increased gradually as needed and tolerated.
