Drug Catalog - Product Detail
CEVIMELINE HCL CP 30MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16571-0657-10 | RISING PHARMA HOLDINGS | 100 | 30MG | CAPSULE |
PACKAGE FILES

Generic Name
CEVIMELINE HYDROCHLORIDE
Substance Name
CEVIMELINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203775
Description
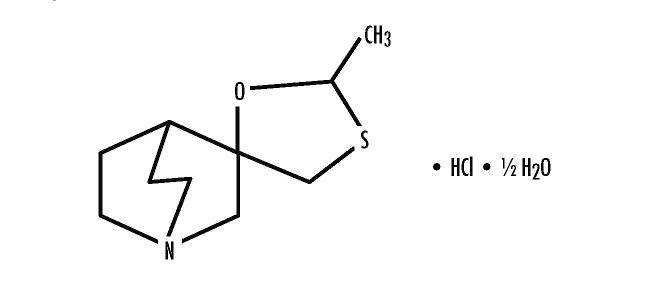
DESCRIPTION Cevimeline is cis-2’-methylspiro {1-azabicyclo [2.2.2] octane-3, 5’ -[1,3] oxathiolane} hydrochloride, hydrate (2:1). Its empirical formula is C 10 H 17 NOS.HCl.1/2 H 2 O, and its structural formula is: Cevimeline has a molecular weight of 244.79. It is a white to off white crystalline powder with a melting point range of 201 to 203°C. It is freely soluble in alcohol and chloroform, very soluble in water, and virtually insoluble in ether. The pH of a 1% solution ranges from 4.6 to 5.6. Inactive ingredients include lactose monohydrate, hydroxypropyl cellulose, and magnesium stearate. Empty capsule shell consists of Titanium Dioxide and Gelatin. Ink used in the imprint is Black SW-9049 which contains Shellac, Dehydrated alcohol, Isopropyl Alcohol, Butyl Alcohol, Propylene Glycol, Purified Water, Strong Ammonia Solution, Potassium Hydroxide, and Black Iron Oxide. Structural Formula
How Supplied
HOW SUPPLIED Cevimeline Hydrochloride Capsules, 30 mg are available as white, hard gelatin capsules containing 30 mg of Cevimeline Hydrochloride. Cevimeline Hydrochloride Capsules are size 3 hard gelatin capsules with an opaque white cap and body. Cevimeline Hydrochloride capsules are imprinted with P 657 on both cap and body in black ink. Cevimeline Hydrochloride is supplied in child resistant bottles of: 100 capsules (NDC: 16571-657-10) Store at 25°C (77°F) excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Rx only Manufactured for: Rising Pharma Holdings, Inc. East Brunswick, NJ 08816 Rev. 07/24 551604 PIR65710-02
Indications & Usage
INDICATIONS AND USAGE Cevimeline is indicated for the treatment of symptoms of dry mouth in patients with Sjögren’s Syndrome.
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended dose of Cevimeline Hydrochloride is 30 mg taken three times a day. There is insufficient safety information to support doses greater than 30 mg tid. There is also insufficient evidence for additional efficacy of Cevimeline Hydrochloride at doses greater than 30 mg tid.
