Drug Catalog - Product Detail
CELECOXIB 400MG CAP 60CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0420-03 | CIPLA USA | 60 | 400MG | CAPSULE |
PACKAGE FILES

Generic Name
CELECOXIB
Substance Name
CELECOXIB
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207446
Description
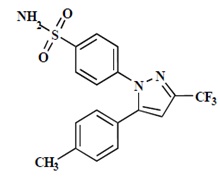
11 DESCRIPTION Celecoxib is a nonsteroidal anti-inflammatory drug, available as capsules containing 50 mg, 100 mg, 200 mg and 400 mg celecoxib for oral administration. The chemical name is 4-[5-(4-methylphenyl)- 3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl-substituted pyrazole. The molecular weight is 381.38. Its molecular formula is C 17 H 14 F 3 N 3 O 2 S, and it has the following chemical structure: Celecoxib is a white to off-white powder with a pKa of 11.1 (sulfonamide moiety). Celecoxib is hydrophobic (log P is 3.5) and is practically insoluble in aqueous media at physiological pH range. The inactive ingredients in celecoxib capsules include: croscarmellose sodium, edible inks, gelatin, lactose monohydrate, magnesium stearate, povidone and sodium lauryl sulfate. image
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Celecoxib Capsules are available in the following strengths and configurations: Product Name Description Size NDC number Celecoxib Capsules 50 mg White to off white colored granules filled in size 2 hard gelatin white capsule, axially printed with 'Cipla' on cap & '423' over '50 mg' on body in red ink. Bottle of 30 capsules Bottle of 60 capsules 69097-423-02 69097-423-03 Celecoxib Capsules 100 mg White to off white colored granules filled in size 2 hard gelatin white capsule, axially printed with 'Cipla' on cap & '422' over '100 mg' on body in blue ink. Bottle of 30 capsules Bottle of 100 capsules Bottle of 500 capsules 10's Blister Carton of 100 (10 x 10) Unit- dose Capsules 69097-422-02 69097-422-07 69097-422-12 69097-422-19 69097-422-21 Celecoxib Capsules 200 mg White to off white colored granules filled in size 1 hard gelatin white capsule, axially printed with 'Cipla' on cap & '421' over '200 mg' on body in gold ink. Bottle of 30 capsules Bottle of 100 capsules Bottle of 500 capsules 10's Blister Carton of 100 (10 x 10) Unit- dose Capsules 69097-421-02 69097-421-07 69097-421-12 69097-421-19 69097-421-21 Celecoxib Capsules 400 mg White to off white colored granules filled in size 0el hard gelatin white capsule, axially printed with 'Cipla' on cap & '420' over '400 mg' on body in green ink. Bottle of 30 capsules Bottle of 60 capsules Bottle of 480 capsules 10's Blister Carton of 100 (10 x 10) Unit- dose Capsules 69097-420-02 69097-420-03 69097-420-11 69097-420-19 69097-420-21 Storage: Store at 25°C (77°F) excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Celecoxib is a non-steroidal anti-inflammatory drug indicated for: Osteoarthritis (OA) ( 1.1 ) Rheumatoid Arthritis (RA) ( 1.2 ) Juvenile Rheumatoid Arthritis (JRA) in patients 2 years and older ( 1.3 ) Ankylosing Spondylitis (AS) ( 1.4 ) Acute Pain (AP) ( 1.5 ) Primary Dysmenorrhea (PD) ( 1.6 ) Celecoxib capsules are indicated 1.1 Osteoarthritis For the management of the signs and symptoms of OA [ see Clinical Studies ( 14.1 ) ]. 1.2 Rheumatoid Arthritis For the management of the signs and symptoms of RA [ see Clinical Studies ( 14.2 ) ]. 1.3 Juvenile Rheumatoid Arthritis For the management of the signs and symptoms of JRA in patients 2 years and older [ see Clinical Studies ( 14.3 ) ]. 1.4 Ankylosing Spondylitis For the management of the signs and symptoms of AS [ see Clinical Studies ( 14.4 ) ]. 1.5 Acute Pain For the management of acute pain in adults [ see Clinical Studies ( 14.5 ) ]. 1.6 Primary Dysmenorrhea For the management of primary dysmenorrhea [see Clinical Studies ( 14.5 ) ].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. ( 2.1 ) OA: 200 mg once daily or 100 mg twice daily. ( 2.2 , 14.1 ) RA: 100 mg to 200 mg twice daily. ( 2.3 , 14.2 ) JRA: 50 mg twice daily in patients 10 kg to 25 kg. 100 mg twice daily in patients more than 25 kg. ( 2.4 , 14.3 ) AS: 200 mg once daily single dose or 100 mg twice daily. If no effect is observed after 6 weeks, a trial of 400 mg (single or divided doses) may be of benefit. ( 2.5 , 14.4 ) AP and PD: 400 mg initially, followed by 200 mg dose if needed on first day. On subsequent days, 200 mg twice daily as needed. ( 2.6 , 14.5 ) Hepatic Impairment: Reduce daily dose by 50% in patients with moderate hepatic impairment (Child-Pugh Class B). ( 2.7 , 8.6 , 12.3 ) Poor Metabolizers of CYP2C9 Substrates: Consider a dose reduction by 50% (or alternative management for JRA) in patients who are known or suspected to be CYP2C9 poor metabolizers, ( 2.7 , 8.8 , 12.3 ). 2.1 General Dosing Instructions Carefully consider the potential benefits and risks of celecoxib capsules and other treatment options before deciding to use celecoxib capsules. Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [ see Warnings and Precautions ( 5 ) ]. These doses can be given without regard to timing of meals. 2.2 Osteoarthritis For OA, the dosage is 200 mg per day administered as a single dose or as 100 mg twice daily. 2.3 Rheumatoid Arthritis For RA, the dosage is 100 mg to 200 mg twice daily. 2.4 Juvenile Rheumatoid Arthritis For JRA, the dosage for pediatric patients (age 2 years and older) is based on weight. For patients ≥10 kg to 25 kg the recommended dose is 50 mg twice daily. For patients >25 kg the recommended dose is 100 mg twice daily. For patients who have difficulty swallowing capsules, the contents of a celecoxib capsule can be added to applesauce. The entire capsule contents are carefully emptied onto a level teaspoon of cool or room temperature applesauce and ingested immediately with water. The sprinkled capsule contents on applesauce are stable for up to 6 hours under refrigerated conditions (2°C to 8°C/35°F to 45°F). 2.5 Ankylosing Spondylitis For AS, the dosage of celecoxib capsules is 200 mg daily in single (once per day) or divided (twice per day) doses. If no effect is observed after 6 weeks, a trial of 400 mg daily may be worthwhile. If no effect is observed after 6 weeks on 400 mg daily, a response is not likely and consideration should be given to alternate treatment options. 2.6 Management of Acute Pain and Treatment of Primary Dysmenorrhea For management of Acute Pain and Treatment of Primary Dysmenorrhea, the dosage is 400 mg initially, followed by an additional 200 mg dose if needed on the first day. On subsequent days, the recommended dose is 200 mg twice daily as needed. 2.7 Special Populations Hepatic Impairment In patients with moderate hepatic impairment (Child-Pugh Class B), reduce the dose by 50%. The use of celecoxib capsules in patients with severe hepatic impairment is not recommended [see Warnings and Precautions ( 5.3 ), Use in Specific Populations ( 8.6 ), and Clinical Pharmacology ( 12.3 )]. Poor Metabolizers of CYP2C9 Substrates In adult patients who are known or suspected to be poor CYP2C9 metabolizers based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin, phenytoin), initiate treatment with half of the lowest recommended dose. In patients with JRA who are known or suspected to be poor CYP2C9 metabolizers, consider using alternative treatments [see Use in Specific populations ( 8.8 ) and Clinical Pharmacology ( 12.5 )].
